Methods for treating infectious disease exacerbated asthma
An aggravating, infectious disease technology, applied in biochemical equipment and methods, antiviral agents, pharmaceutical formulations, etc., can solve problems such as weak activation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
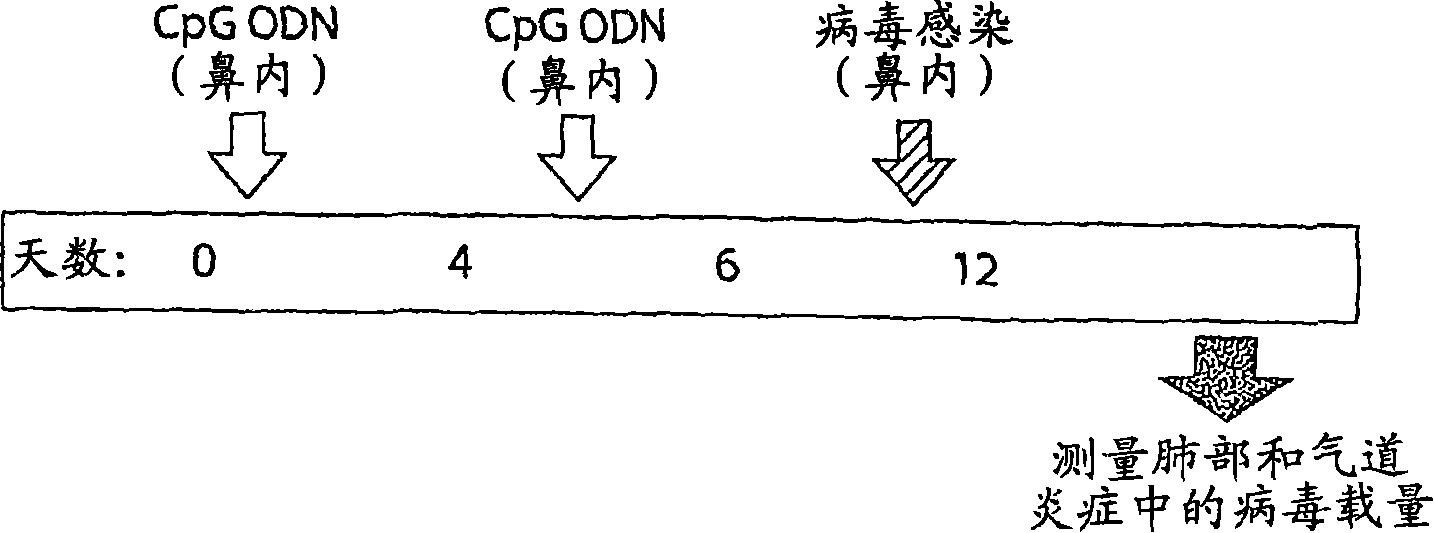
[0203] 1. Induction of IFNα and IFN-related genes by CpG ODN (SEQ ID NO: 10)
[0204] method:
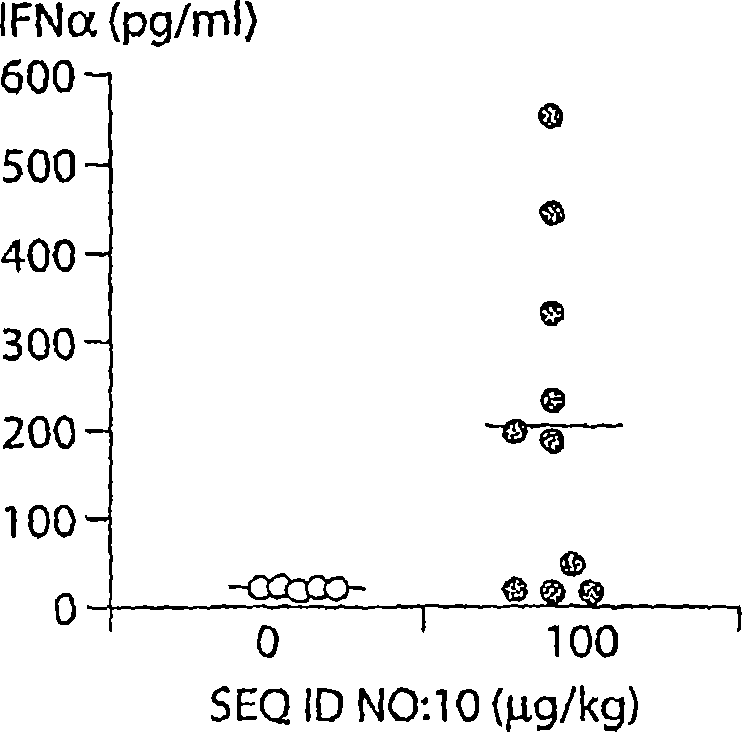
[0205] Mice (male BALB / c) received SEQ ID NO: 10 ( Figure 3a-3c The medium is 100μg / kg, Figure 3d-3f Medium is 10, 100 or 1000μg / kg) or saline. After 15 hours, analyze the secreted proteins (IFN α, IFN γ and IP10) in bronchial lavage fluid, or analyze the gene expression in lung tissue by real-time PCR after 30 hours.
[0206] result:
[0207] Class C CpG ODN induced the secretion of IFNα, IFNγ, and interferon-induced protein 10 (IP-10). The results are shown in Figure 3.
[0208] Because CpG ODN stimulated the secretion of IFNα in the airways of mice, we investigated whether the interferon-inducible indoleamine 2, 3 dioxygenase gene is expressed in the lungs. When CpG ODN was instilled into the airway, it did increase the expression of this immunomodulatory enzyme mRNA ( Figure 3f ). It also up-regulated Mx1 and indoleamine 2, 3 dioxygenase in the lungs of mice ( Figure 3d with 3e ). ...
Embodiment 2
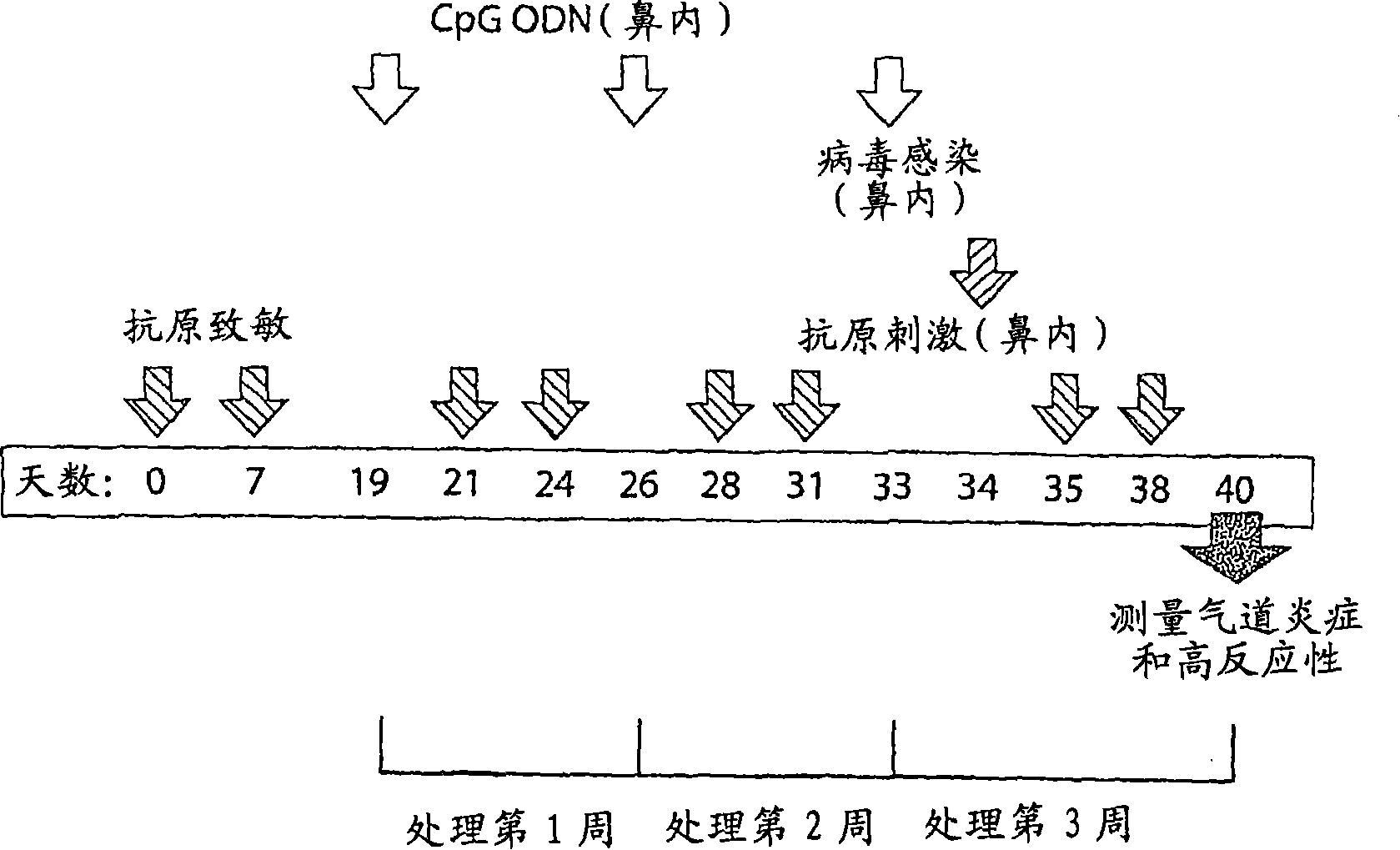
[0226] We have shown that CpG oligodeoxynucleotides can inhibit mouse influenza virus load and virus-induced airway inflammation. In Example 2, we studied the protective effect of SEQ ID NO: 10 against the exacerbated airway inflammation induced by the combination of influenza virus infection and antigen stimulation.
[0227] method:
[0228] 1. Antigen and virus administration:
[0229] Mice (male BALB / c) were sensitized with antigen (cockroach, 10 μg, intraperitoneal) and aluminum hydroxide adjuvant (Pierce Alum) on day 0 and day 7 of the study.
[0230] The mice were subjected to antigen stimulation by contacting the intranasally administered antigen (10 μg, 40 μl saline solution) twice a week for three consecutive weeks. The first stimulation was performed on the 21st day of the study.
[0231] On the 34th day of the study (that is, before the last two antigen stimulations), the influenza virus (type A influenza, H1N1 subtype, mouse-adapted strain PR8, 200 EID) was injected i...
Embodiment 3
[0250] Induction of TLR9-related cytokines from mouse spleen cells in vitro and in vivo mouse lungs
[0251] The ability of SEQ ID NO: 10 to induce murine splenocytes to secrete TLR9-related cytokines in vitro was studied.
[0252] method
[0253] Stimulating cytokines from splenocytes in vitro
[0254] Spleen cells from 6 mice were pooled and incubated with ODN (0.1, 1, or 10 μg / ml) for 36 hours. The crushed mouse spleen was gently squeezed through a cell sieve (pore size 70 μm), and the cells were mechanically separated. Incubate in 1ml medium (RPMI 1640 containing 10% fetal bovine serum, all from Invitrogen, Carlsbad, CA, USA) (37°C, 5% CO 2 ) Cell (1×10 7 , Taken from 6 mice). Add SEQ ID NO: 10 or control ODN (with reverse CpG motif) or any one of the two domains of SEQ ID NO: 10 (5' terminal stimulation sequence and palindrome), the given concentration is 0.1, 1 or 10μg / ml. After 24 hours of incubation, the secreted cytokines (IFNα, IFNγ, interferon-inducing protein [IP]-10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com