Utilization method of solid catalyst for cyclohexanone self-condensation reaction
A technology of solid catalyst and condensation reaction, applied in chemical instruments and methods, preparation of organic compounds, physical/chemical process catalysts, etc., can solve problems such as large limitations, deactivation, and poor catalyst activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
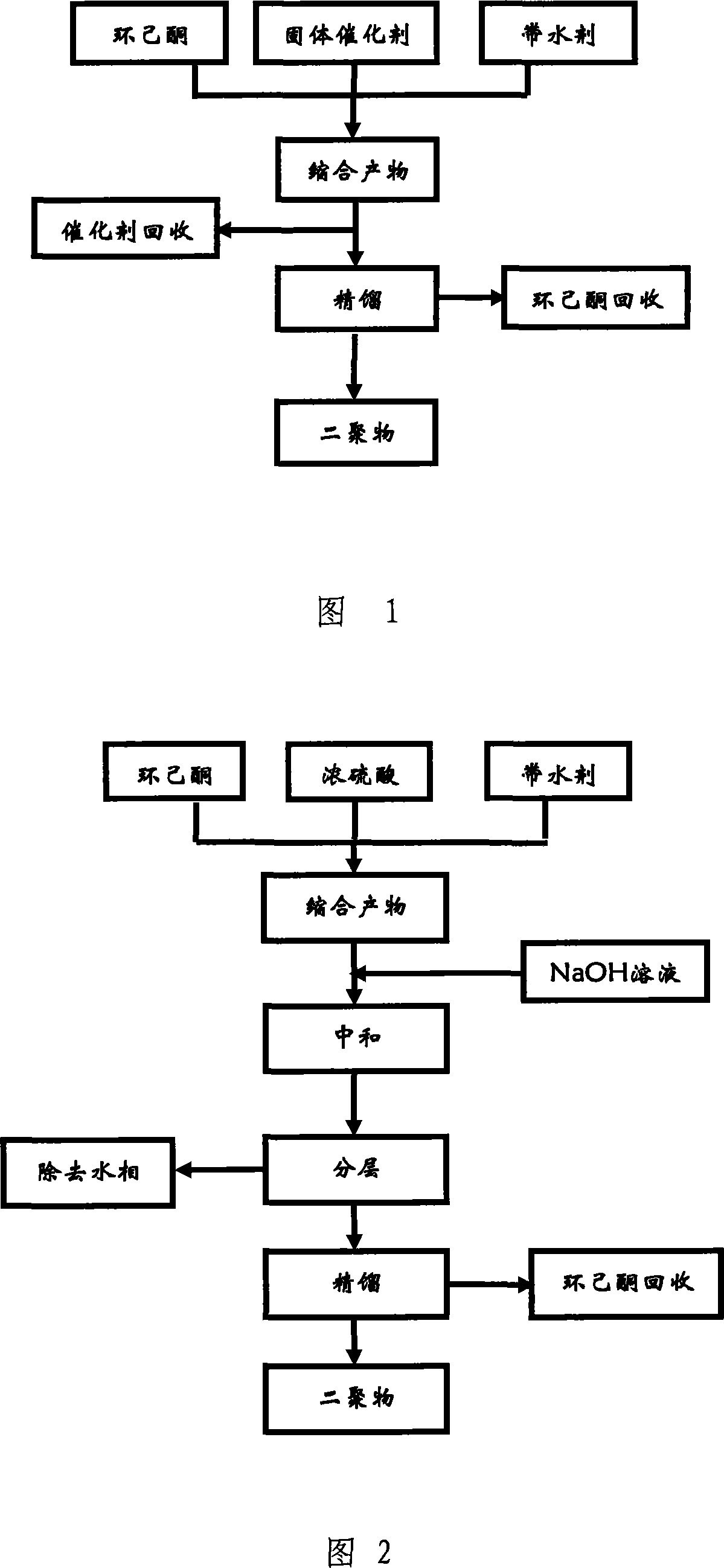
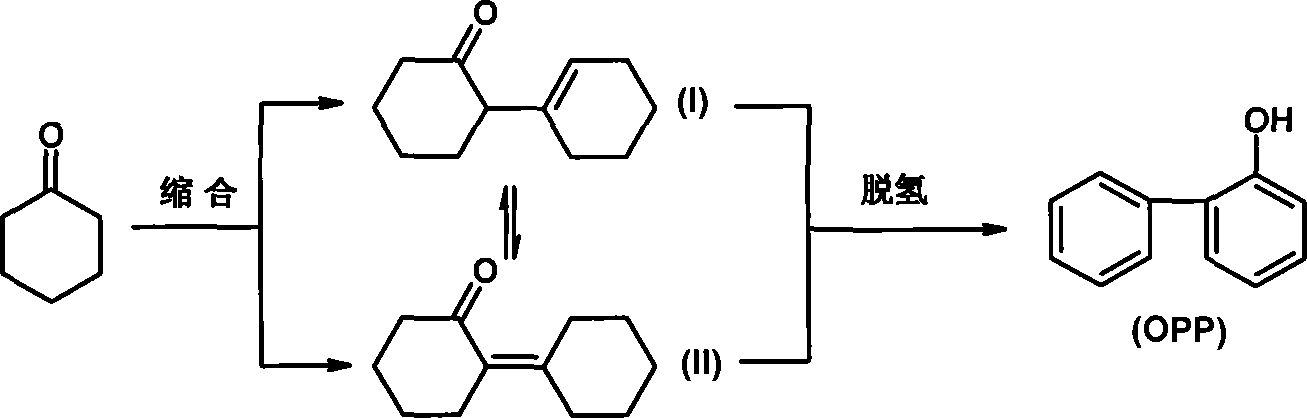
[0021] This example is a comparative example of concentrated sulfuric acid catalyst. Put 100mL of cyclohexanone into a three-neck flask equipped with a thermometer, agitator and water separator, add 2mL of 98% concentrated sulfuric acid as a catalyst, and 60mL of cyclohexane as a water-carrying agent, reflux at 110°C for 2 hours, and the reaction stops Finally, the reaction system was adjusted to neutral with 10% NaOH solution, and after the water layer was separated, the oil phase was taken for chromatographic analysis. The conversion rate of cyclohexanone was about 85.7%, the selectivity was about 83.5%, and the dimer yield was 71.6%. .
Embodiment 2
[0023] Put 100mL of cyclohexanone in a three-neck flask equipped with a thermometer, agitator and water separator, add water in n-heptane (amount of 60mL), add 4g of γ-Al obtained by roasting at 500°C for 4 hours in advance 2 o 3 Catalyst (pore volume 0.52mL / g, specific surface area 207m 2 / g), heated to system boiling reflux reaction for 6 hours, cyclohexanone conversion rate 75.7%, dimer selectivity 99.0%, dimer yield 74.9%; water-carrying agent is unchanged, and replacement catalyst is 5% K 2 O-Al 2 o 3 Composite catalyst, cyclohexanone conversion rate 82.1%, dimer selectivity 96.6%, dimer yield 79.3%; the water-carrying agent remains unchanged, and the catalyst is replaced with 10% TiO 2 -Al 2 o 3Compound catalyst catalyst, cyclohexanone conversion rate 78.3%, dimer selectivity 98.6%, dimer yield 77.2%; catalyst is γ-Al 2 o 3 , replacing the water-carrying agent with toluene (consumption unchanged), the conversion rate of cyclohexanone is 88.8%, the dimer selectivit...
Embodiment 3
[0025] Put 100mL of cyclohexanone into a three-neck flask equipped with a thermometer, agitator and water separator, add water in n-heptane (amount of 60mL), add 4g of industrial macroporous Al obtained by roasting at 500°C for 4 hours in advance 2 o 3 Catalyst (pore volume 0.79mL / g, specific surface 281m 2 / g), heated until the system boiled and refluxed for 6 hours, the conversion rate of cyclohexanone was 81.8%, the dimer selectivity was 96.5%, and the dimer yield was 78.9%. Other conditions remain unchanged, the replacement catalyst is 33.5% MgO-macroporous Al 2 o 3 With the compound catalyst, the conversion rate of cyclohexanone is 76.5%, the dimer selectivity is 95.2%, and the dimer yield is 72.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com