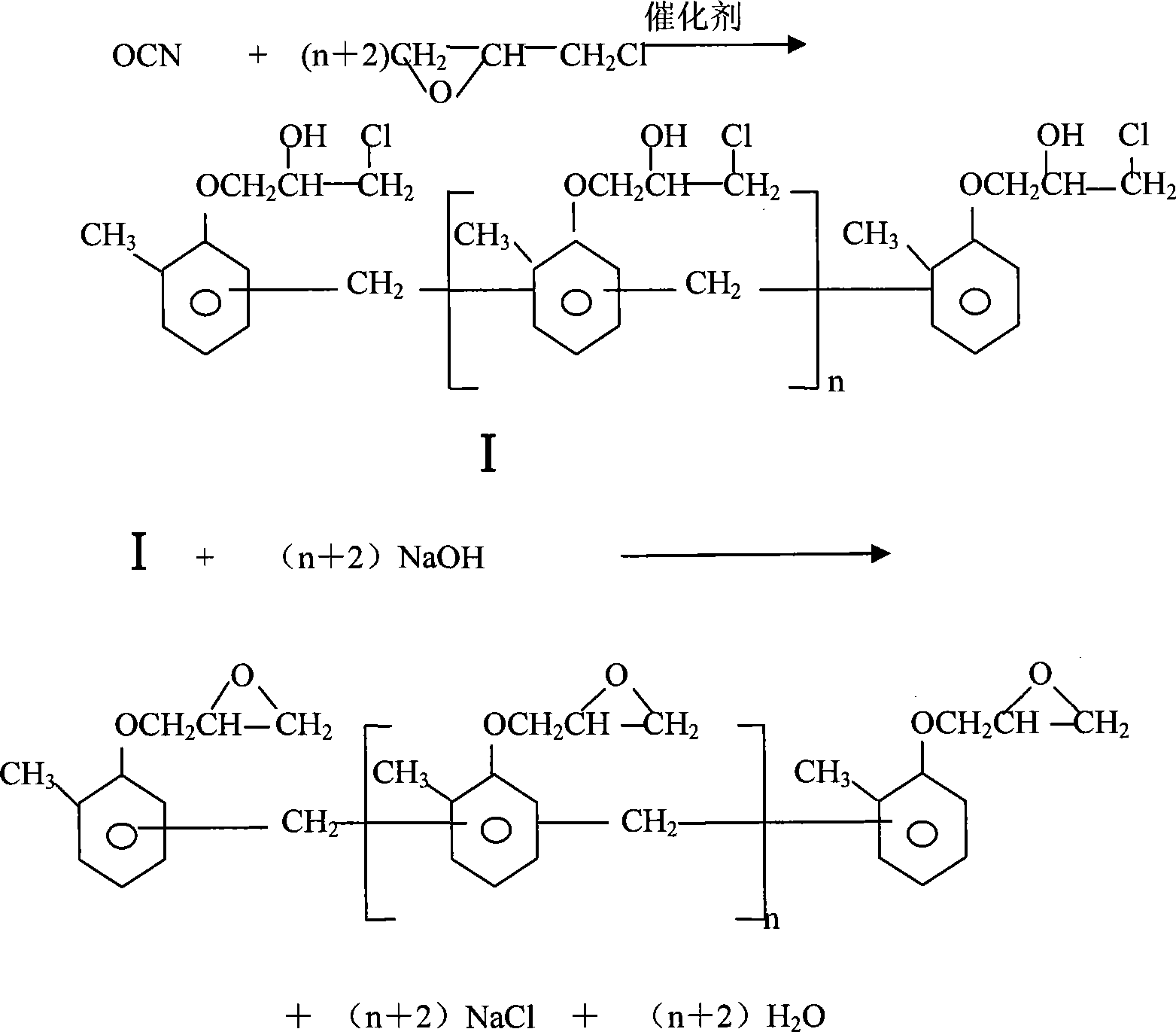
Synthesis of o-cresol formaldehyde epoxy resin
A novolac epoxy resin and a synthesis method technology, which is applied in the field of synthesis of o-cresol novolac epoxy resin, can solve the problem of no inert gas being injected into the recovered epichlorohydrin, and achieve a stable synthesis method, high epoxy value, The effect of low saponification chlorine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 100g of OCN and 400g of epichlorohydrin into a 5000ml four-neck flask, dissolve at 40°C for 3h at a vacuum of 0.1Mpa; add 0.1g of triethylamine, and etherify at 40°C and at a vacuum of 0.02MPa for 3h; At 40°C and a vacuum of 0.02MPa, 120g of 50% sodium hydroxide solution was added dropwise, and the addition was completed in 3 hours. The refluxed epichlorohydrin flowed back to the reactor in time to maintain the closed-loop reaction for 2 hours; the epoxychlorohydrin was recovered by heating to At 120°C, feed inert gas, adjust the flow rate of the inert gas according to the vacuum degree, keep the vacuum degree at 0.06Mpa, pass the inert gas for 1 hour, turn off the inert gas, and continue to recover until the final temperature is 130°C, and the vacuum degree is greater than 0.09Mpa; the recovery is completed Add 500g of toluene to dissolve for 0.5h, add 100g of 10% sodium hydroxide solution to refine and react for 5h at 75°C under normal pressure; add 300g of toluene...
Embodiment 2
[0037] Change the sodium hydroxide solution concentration of refining reaction into 30% in example 1, other conditions are constant, obtain EOCN139g.
[0038] It is determined that the softening point of the EOCN obtained in this example is 87° C., the epoxy equivalent is 195 g / eq, and the easily saponifiable chlorine is 455 ppm.
Embodiment 3
[0040] Add 100g of OCN and 600g of epichlorohydrin into a 5000ml four-neck flask, dissolve at 100°C for 0.5h at a vacuum of 0.05MPa; add 0.1g of benzyltriethylammonium chloride and 8g of 50% sodium hydroxide solution , Etherification at 75°C and a vacuum of 0.05MPa for 0.5h; dropwise adding 90g of 48% potassium hydroxide solution at 75°C and a vacuum of 0.05MPa for 3h, and maintain the ring closure reaction for 2h; heat to recover epichlorohydrin When the temperature reaches 125°C, feed the inert gas, adjust the flow rate of the inert gas according to the vacuum degree, keep the vacuum degree at 0.8Mpa, pass the inert gas for 2 hours, turn off the inert gas, and continue to recover until the final temperature is 200°C, and the vacuum degree is greater than 0.09Mpa; After the recovery, add 200g of butanol to dissolve for 1.5h, add 40g of 25% potassium hydroxide solution to undergo a refining reaction at 80°C and normal pressure for 3h; add 900g of xylene, then add 200g of deioni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Softening point | aaaaa | aaaaa |
| Epoxy equivalent | aaaaa | aaaaa |
| Epoxy equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com