Percutaneous absorption patch containing gestodene and/or estrogen
A gestodene and estrogen technology, applied in the field of pharmaceutical preparations, can solve the problem of not mentioning gestodene, etc., and achieve the effects of improving compliance and ensuring contraceptive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Composition in the first layer (outer layer): polyvinyl alcohol (PVA17-88) 4.5g, polyvinylpyrrolidone (PVP) 0.5g, azone (Azone) 1.0g, propylene glycol 0.2g, glycerin 1.0g, pregnane Ketone (GSD) 26.83mg, ethinyl estradiol (EE) 5.3mg.
[0040] The composition of the second layer (the inner layer close to the protective film): polyvinyl alcohol (PVA17-88) 6g, polyvinylpyrrolidone (PVP) 1g, azone (Azone) 2g, propylene glycol 0.3g, glycerin 1.5g , gestodene (GSD) 6.5mg, ethinyl estradiol (EE) 2.5mg.
[0041] Preparation method: fully dissolve gestodene and ethinyl estradiol with 120 g of 50% ethanol, add polyvinyl alcohol, polyvinyl pyrrolidone, etc. to fully swell, add azone and glycerin in a water bath, ultrasonically dissolve, and stand still until the bubbles disappear. Lay the film separately, dry and cool, then compound, cover the backing layer of polyethylene aluminum-polyethylene composite film and polyethylene protective film, and prepare a product containing GSD0....
Embodiment 2
[0044] The composition of the first layer: polyvinyl alcohol (PVA17-88) 4g, polyvinylpyrrolidone (PVP) 0.5g, isopropyl myristate 1g, propylene glycol 0.5g, glycerin 0.6g, GSD 27.8mg, EE 10mg.
[0045] The composition of the second layer: polyvinyl alcohol (PVA17-88) 6g, polyvinylpyrrolidone (PVP) 1.3g, isopropyl myristate 2g, propylene glycol 0.5g, glycerin 0.8g, GSD 6mg, EE 3.0mg.
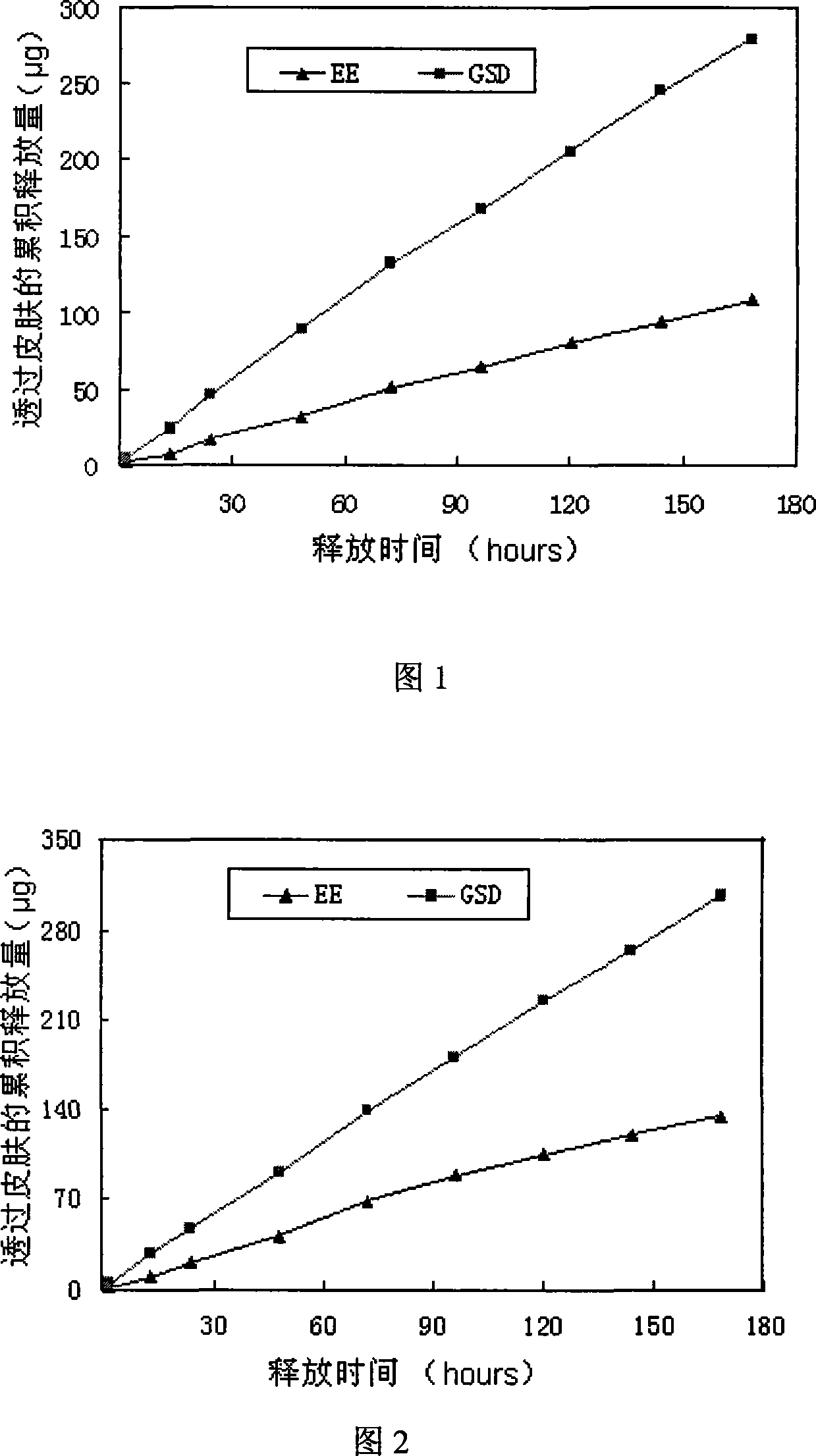
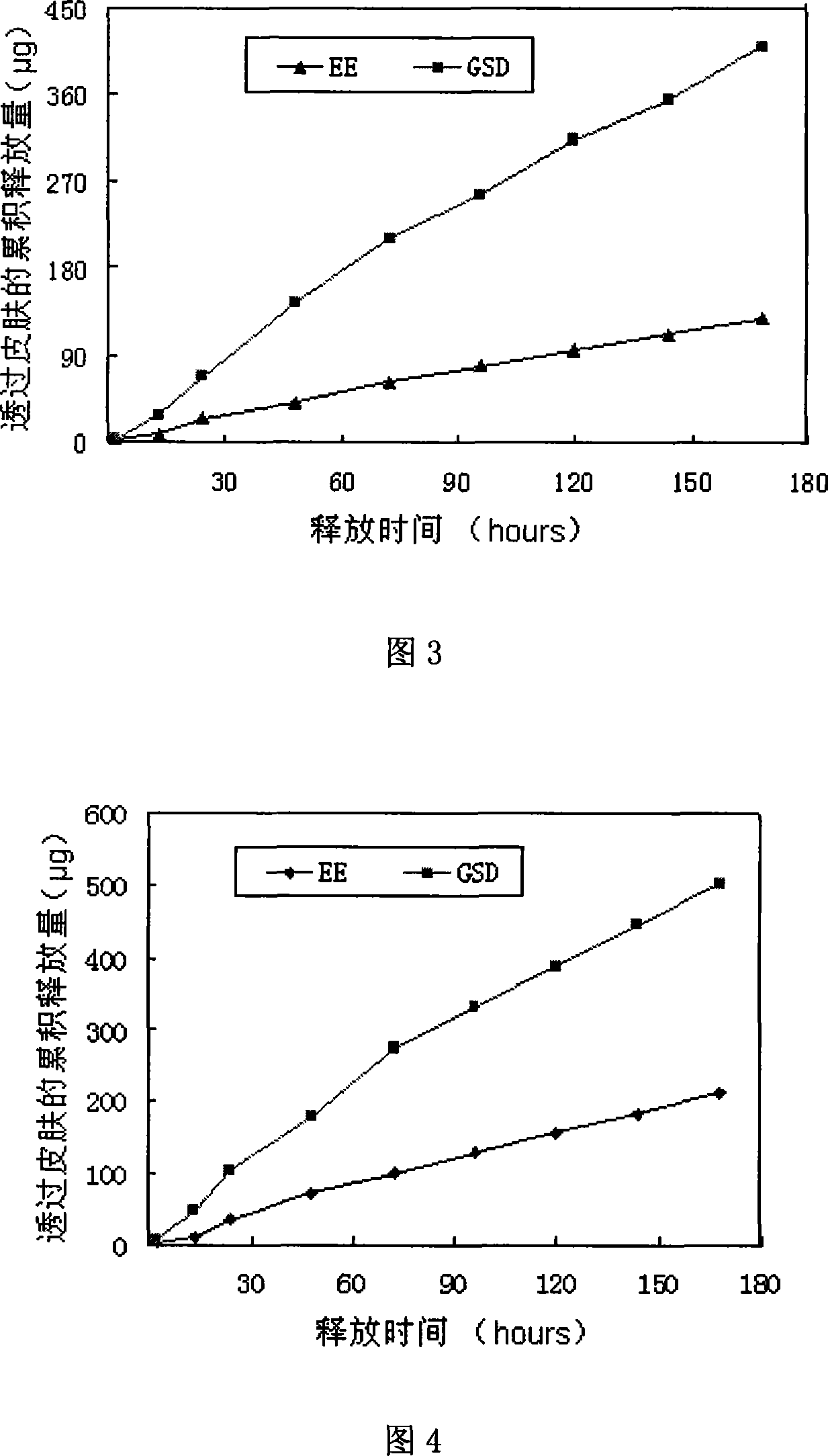
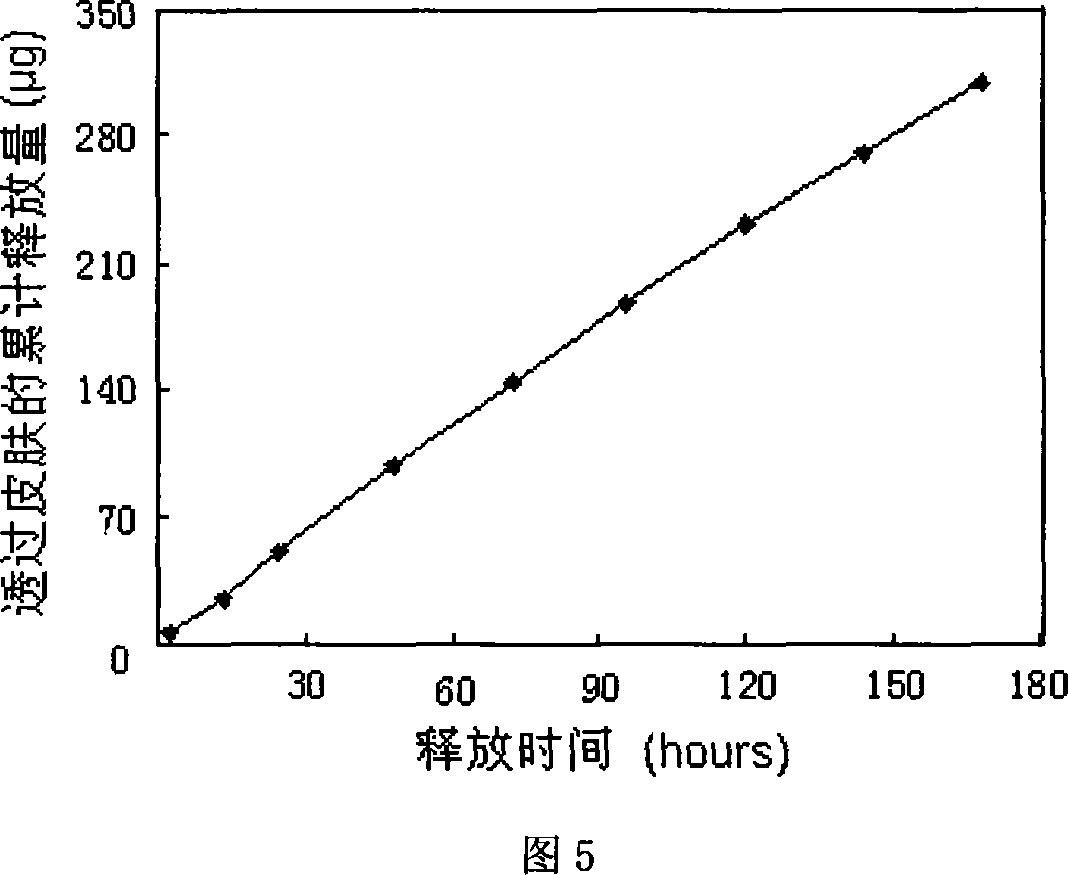
[0046] Prepared as in Example 1, prepared to contain GSD0.3mg / cm 2 , EE0.1mg / cm 2 patch. And carry out transdermal diffusion test on it, the penetration rate of GSD is 0.61μg / cm 2 / h, the permeation rate of EE is 0.25μg / cm 2 / h. The in vitro skin release curve is shown in Figure 2.
Embodiment 3
[0048] First layer: polyvinyl alcohol (PVA17-88) 9g, polyvinylpyrrolidone (PVP) 2g, glycerin 3g, isopropyl myristate 2g, propylene glycol 1.5g, 48mgGSD and 11.5mgEE.
[0049] Second layer: polyvinyl alcohol (PVA17-88) 6g, polyvinylpyrrolidone (PVP) 1g, glycerin 2g, isopropyl myristate 2g, propylene glycol 0.5g, 12mgGSD and 1.5mgEE.
[0050] The patch preparation method is the same as in Example 1, prepared to contain GSD0.33mg / cm 2 , EE0.08mg / cm 2 patch. The penetration rate of GSD in this patch is 0.8 μg / cm 2 / h, the permeation rate of EE is 0.26μg / cm 2 / h. The release curve through the skin in vitro is shown in Figure 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com