Detection method of liu-wei tonic tablets dual-wavelength fingerprint chromatogram
A Liuwei Dihuang Pill and detection method technology, which is applied in the detection field of Liuwei Dihuang Pill dual-wavelength coverage fusion fingerprint, can solve the problems of mobile phase system and pretreatment complexity, inability to evaluate and control the quality of traditional Chinese medicine, and improve quality control Standard, easy to grasp, guarantee standardized effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
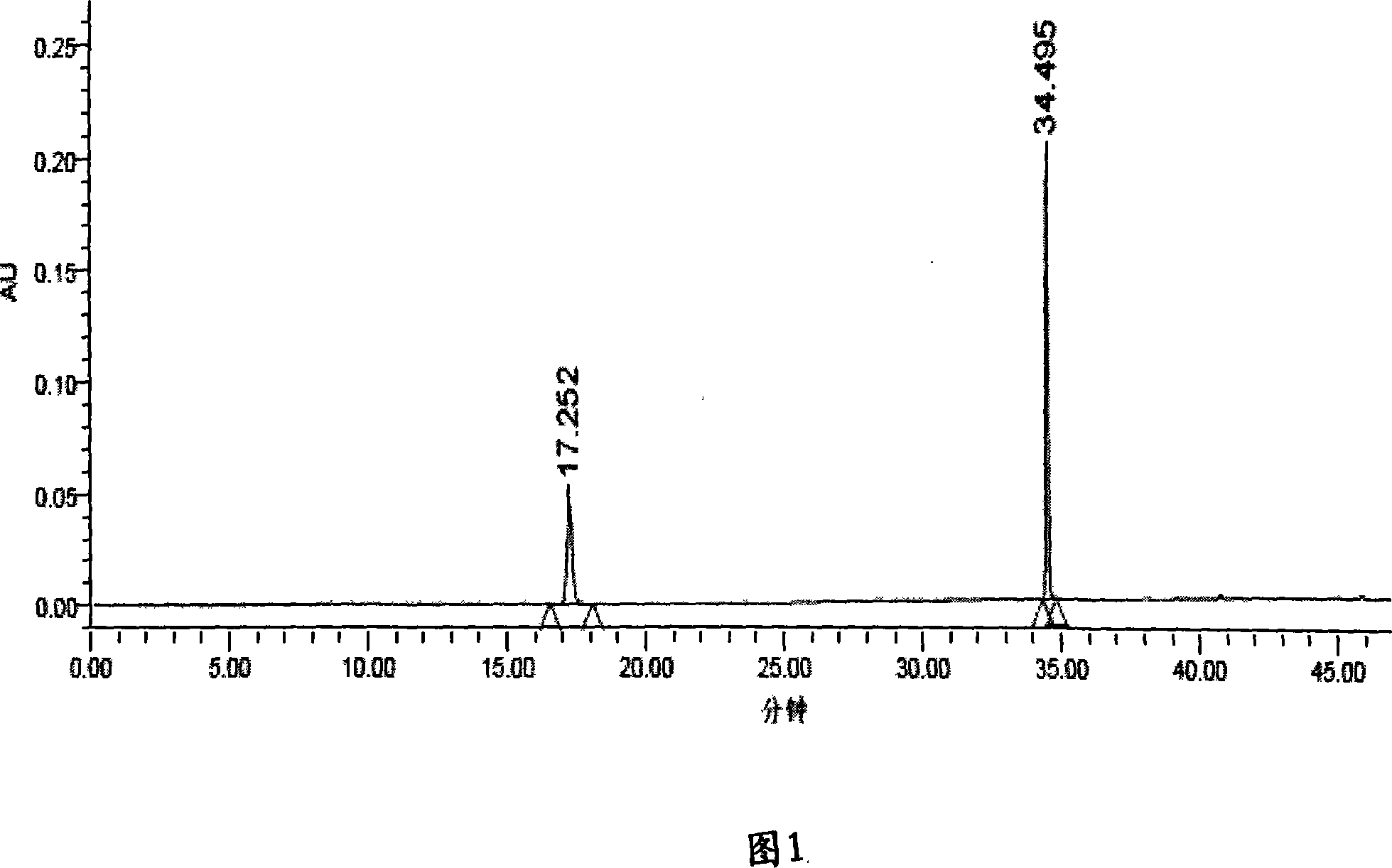
[0040] Take Liuwei Dihuang Pills, chop them into pieces, take 1.0g, accurately weigh them, put them in a stoppered Erlenmeyer flask, add 25mL of methanol precisely, seal them tightly, weigh them, extract them with ultrasound, treat them with ultrasound for 60 minutes, let cool, and weigh again Determine the weight, use methanol to make up the lost weight, shake well, filter, and take the filtrate to obtain the final product.
[0041] Preparation of reference substance solution: Accurately weigh appropriate amounts of loganin reference substance and paeonol reference substance, and add methanol to make solutions containing 14 μg of loganin reference substance and 18 μg of paeonol reference substance per 1 mL, respectively.
[0042] Determination method: According to the conditions of high performance liquid chromatography (Appendix VID of the Chinese Pharmacopoeia 2005 edition). Chromatographic conditions and system suitability test: Chromatographic column: Agilent SB C 18 (4....
Embodiment 2
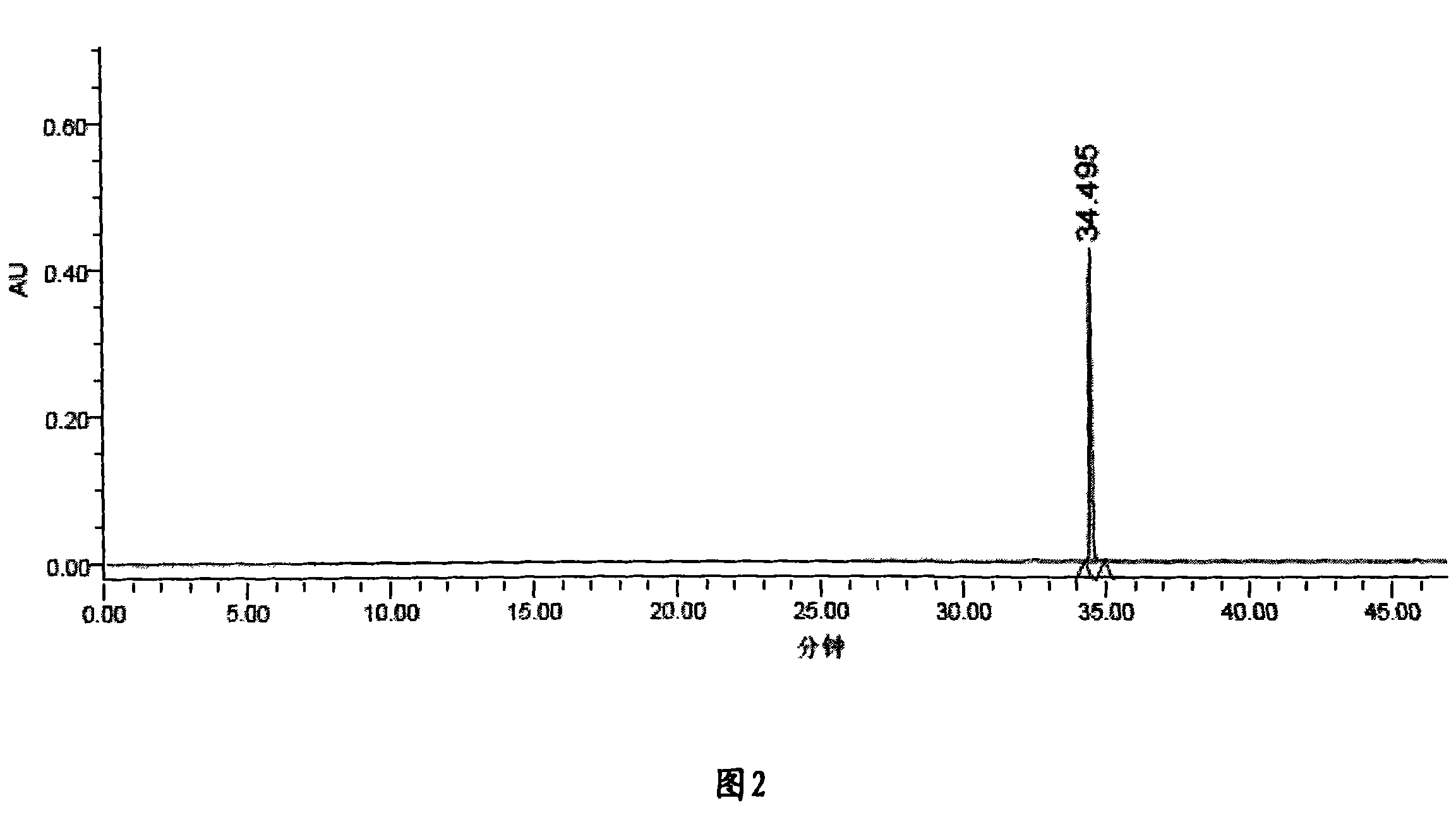
[0050] Take Liuwei Dihuang concentrated pills, chop them up, take 1.0g, weigh them accurately, put them in a stoppered Erlenmeyer flask, add 25mL of methanol precisely, seal them tightly, and weigh them. Ultrasonic extraction is ultrasonic treatment for 120 minutes, let cool, and weigh again. Weight, use methanol to make up the lost weight, shake well, filter, and take the continued filtrate, that is.
[0051] Preparation of reference substance solution: Accurately weigh appropriate amounts of loganin reference substance and paeonol reference substance, and add methanol to make solutions containing 14 μg of loganin reference substance and 18 μg of paeonol reference substance per 1 mL, respectively.
[0052] Determination method: According to the conditions of high performance liquid chromatography (Appendix VID of the Chinese Pharmacopoeia 2005 edition). Chromatographic conditions and system suitability test: Chromatographic column: Agilent SB C 18 (4.6mm×250mm, 5μm); see Tab...
Embodiment 3
[0059] Comparative experiment of the same batch of Liuwei Dihuang pills determined by conventional method and dual-wavelength coverage fusion method:
[0060] normal method:
[0061] Take Liuwei Dihuang Pill sample ××× pharmaceutical company (batch number is B058220) according to the Pharmacopoeia method, respectively assay loganin and paeonol.
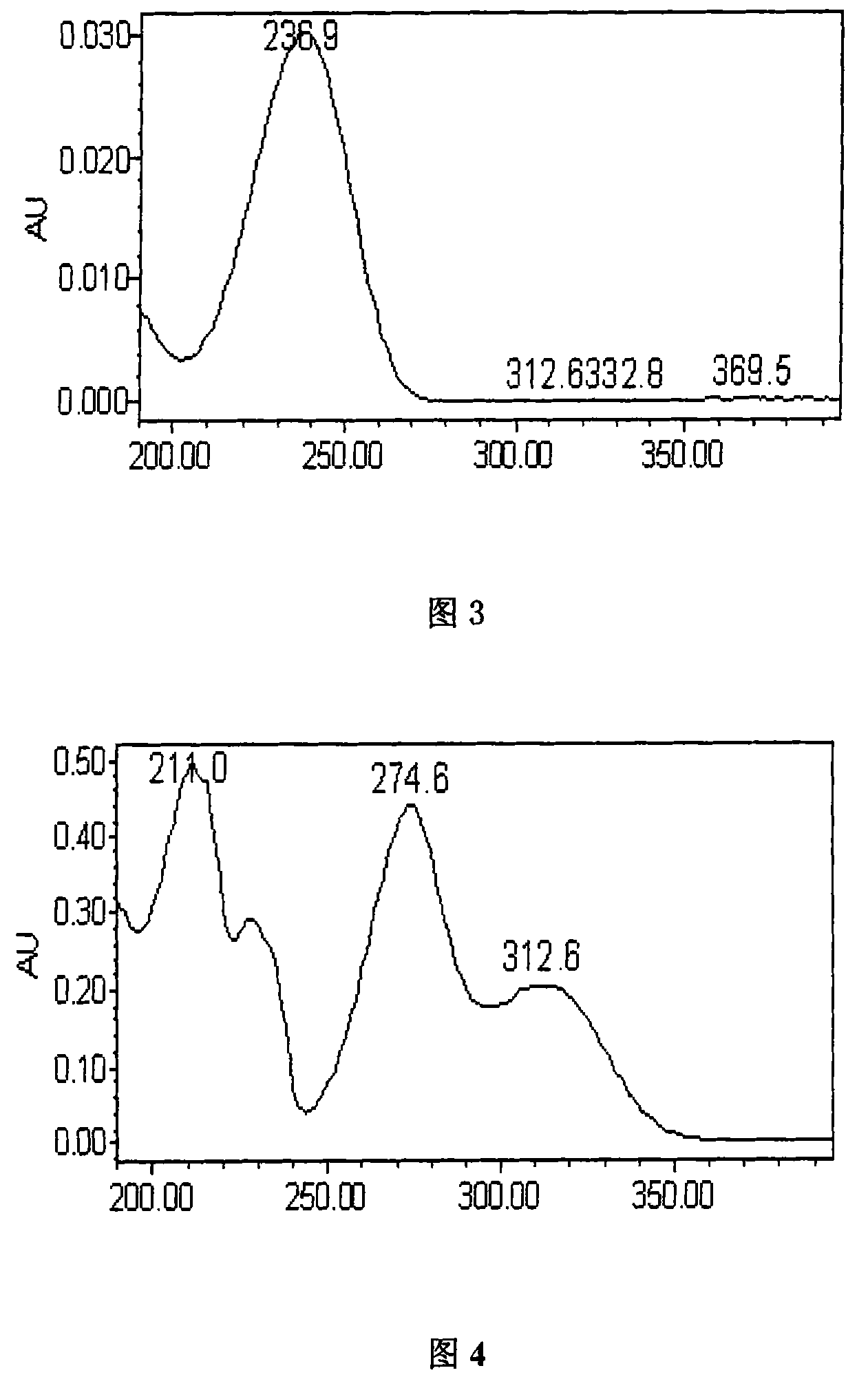
[0062] The assay method of loganin is: chromatographic conditions: using octadecylsilane bonded silica gel as filler; using THF-acetonitrile-methanol-0.05% phosphoric acid solution (1:8:4:87) as mobile phase; detection wavelength is 236nm; the column temperature is 40°C, and the number of theoretical plates should not be less than 3000 based on the loganin peak.
[0063] Preparation of the reference substance solution: Take an appropriate amount of the loganin reference substance, weigh it accurately, add 50% methanol to make a solution containing 20mg per 1mL, and obtain it.
[0064] Preparation of the test solution: take this prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com