Beta-elemene monosubstituted amine derivatives, synthetic method and use thereof
A technology for elemene mono- and synthesis method, which is applied in the field of novel β-elemene mono-substituted amine derivatives and their synthesis, can solve the problems of poor water solubility, limited clinical application and the like, and achieves improved water solubility and anticancer activity. High, high bioavailability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~24
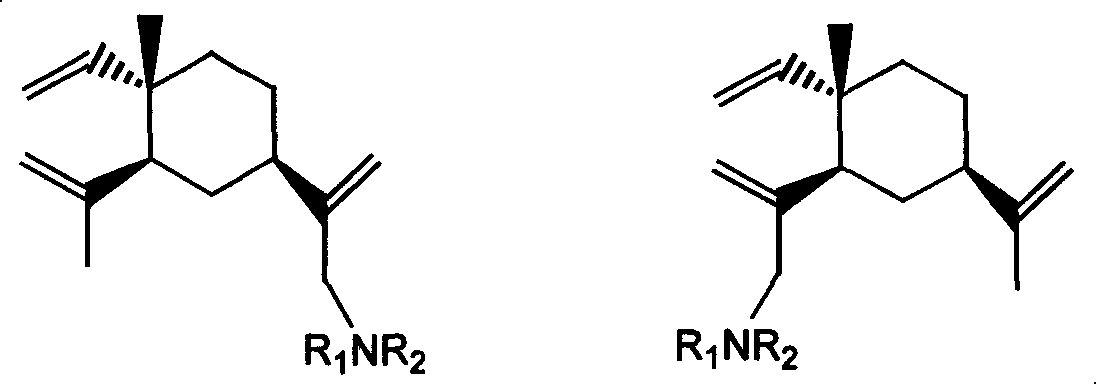
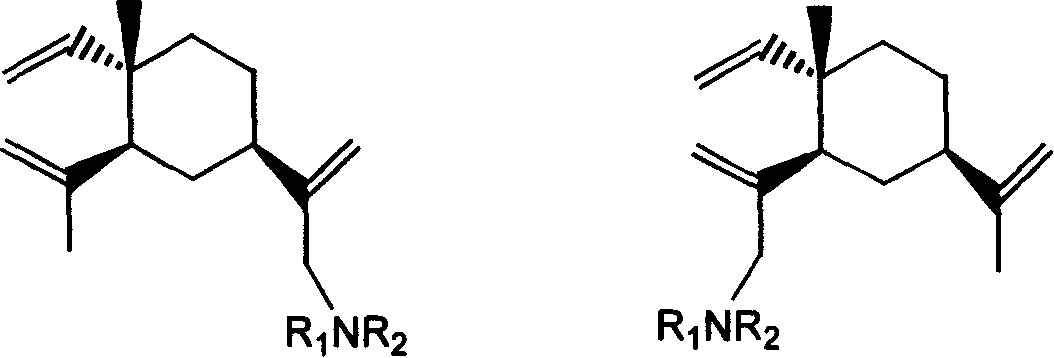
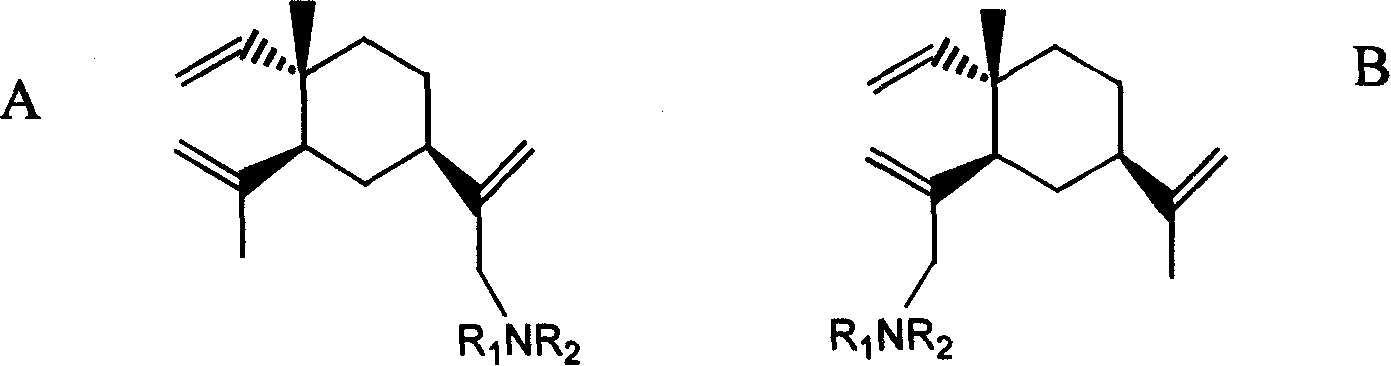
[0030] Embodiment 1~24β-elemene monosubstituted amine derivative
[0031] Table 1 and Table 2 give examples 1 to 24 of β-elemene monosubstituted amine derivatives whose specific structures are A or B in formula I.
[0032]
[0033] Formula I
Embodiment 1~18
[0036] Table 2 Examples 19-24 of β-elemene monosubstituted amine derivatives
[0037]
[0038] The synthesis of reference example β-elemene chloride
[0039] According to the method of literature (Jia Weimin, synthesis, structure and structure-activity research of new anticancer drug β-elemene and its derivatives, doctoral thesis of Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 1991), the method for synthesizing β-elemene chloride, specifically Proceed as follows:
[0040] Dissolve 0.01mol β-elemene in 10mL dichloromethane, add 2mL formic acid, control the temperature at 0-5°C, slowly add 15mL sodium hypochlorite solution dropwise within 2 hours, and continue the reaction for 3-5 hours. After the reaction is complete, add saturated aqueous sodium bicarbonate solution to adjust the pH to 7-8, separate the reaction mixture and separate the organic phase. Sodium drying, filtration, removal of solvent dichloromethane, to obtain a light yellow oil, ...
Embodiment 1
[0043] Synthesis of method embodiment 1 β-elemene monosubstituted cyclohexylamine
[0044]
[0045] Mix 4.5mmol cyclohexylamine, 9mmol β-elemene chloride mixture (containing β-elemene chloride 4.5mmol), 9mmol NaOH and 5mL acetonitrile, and react at 90°C for 2 hours under stirring. After the reaction is completed, cool down, add 20mL saturated brine, then extract 3 times with 10mL dichloromethane, combine the organic phases, and wash the organic matter with anhydrous Na 2 SO 4 Dry, filter, and remove the solvent dichloromethane to obtain a crude product, which can be obtained by silica gel column chromatography and gradient elution with dichloromethane and methanol (volume ratio 20:1). 1 H-NMR identification results are as follows:
[0046] 1 H-NMR (CDCl 3 , TMS, 500MHz), δ0.99(s, 3H), 1.17-1.30(m, 4H), 1.35-1.59(m, 10H), 1.71(s, 3H), 1.78-1.90(m, 2H), 2.05 -2.26(m, 2H), 2.91-3.02(m, 1H), 3.50-3.66(m, 2H), 4.57(s, 1H), 4.82(s, 1H), 4.88(s, 1H), 4.90-4.92 (m, 1H), 5.23 (s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com