Quadridentate-bridged ligand, its iridium complexes and iridium-rare earth ion bimetal complexes, and preparation method and application thereof
A complex and bimetallic technology, applied in the field of rare earth complex luminescent materials, can solve the problems of low luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
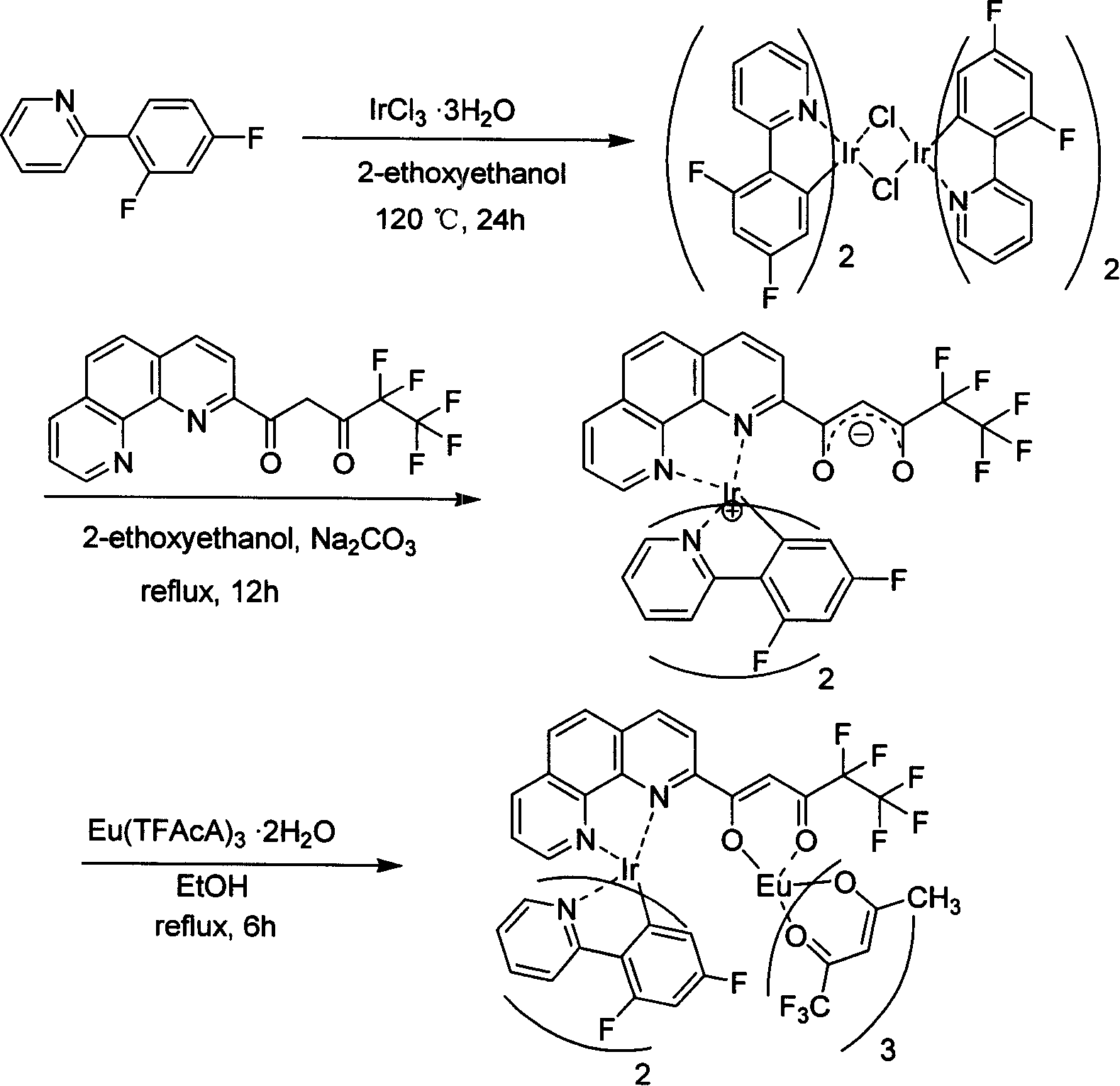
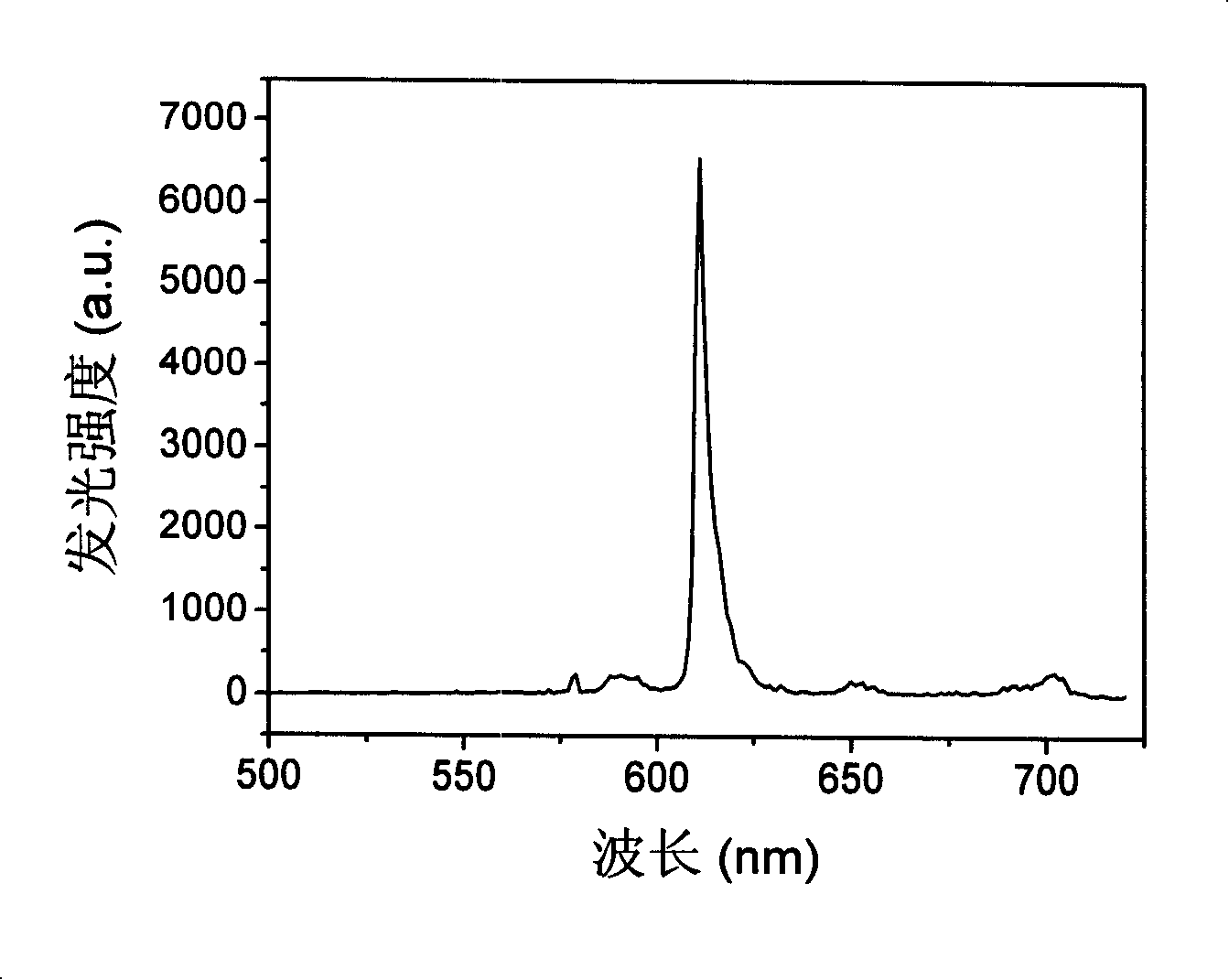
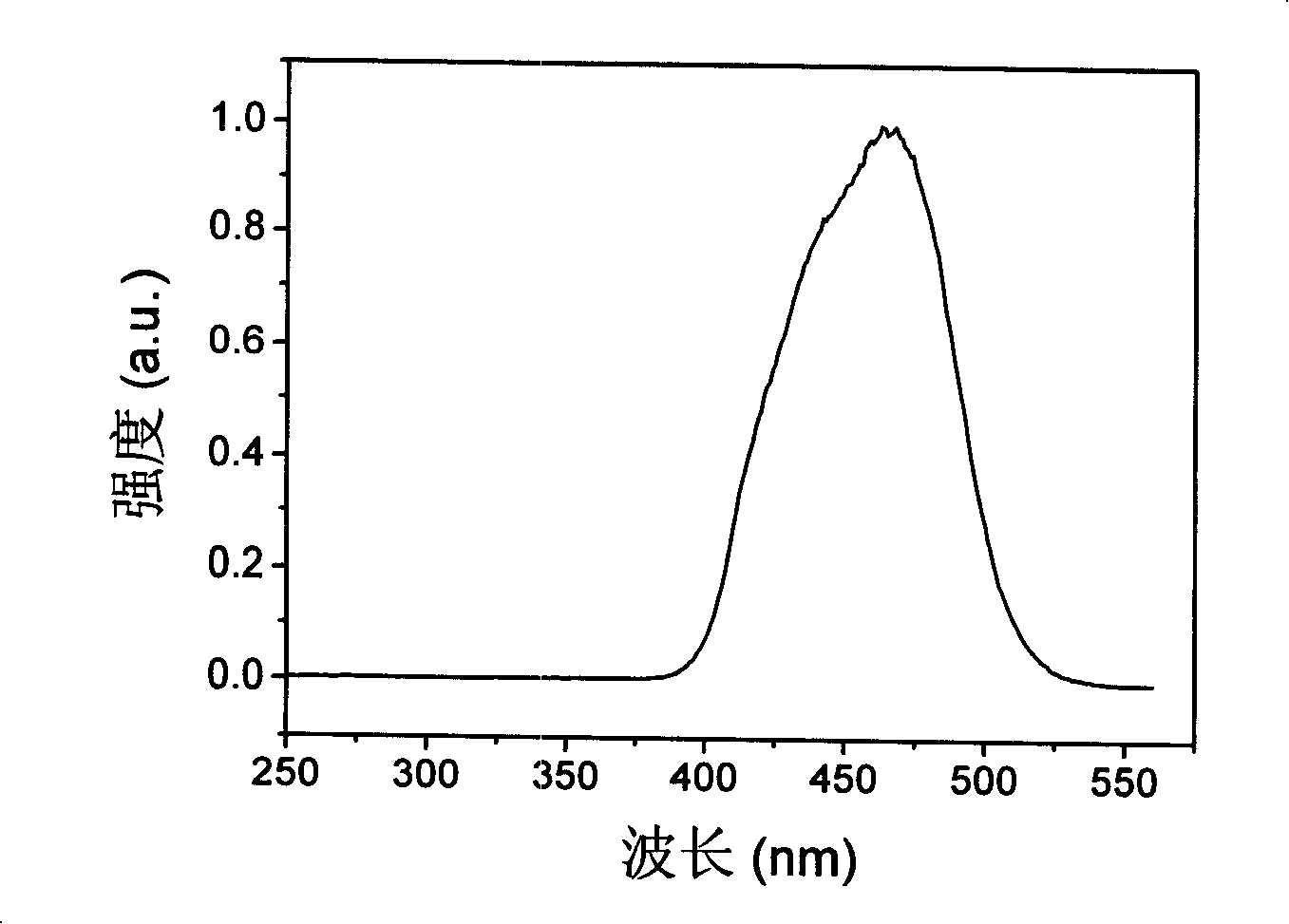
[0070] Example 1. Bridging ligand Hphen5f and double metal complex (dfppy) 2 Ir(μ-phen5f)Eu(TFAcA) 3 Synthesis
[0071] The raw materials used in this embodiment are known compounds, which can be purchased in the market, or can be synthesized by methods known in the art.
[0072] 1. Tetradentate ligand 4,4,5,5,5-pentafluoro-1-(1',10'-o-phenanthroline-2'-yl)-1,3-pentanedione (Hphen5f) Synthetic (1) synthesis of o-phenanthroline nitrogen oxides:
[0073] Add 6.0 mL of 30% hydrogen peroxide dropwise into 60 mL of glacial acetic acid solution containing 10 g of phenanthroline, and react at 70-75° C. for 3 hours. Then 6.0 mL of 30% hydrogen peroxide was added, and the reaction was continued at 70-75° C. for 3 hours. After the reaction, the reaction solution was distilled under reduced pressure to about 15 mL of concentrated solution, and then 15 mL of water was added, and the reaction solution was continued to be distilled under reduced pressure to about 15 mL of concentrated s...
Embodiment 2
[0087] Embodiment two. Double metal complex (dfppy) 2 Ir(μ-phen5f)EuCl 3 Synthesis
[0088] 1. Tetradentate ligand 4,4,5,5,5-pentafluoro-1-(1',10'-o-phenanthroline-2'-yl)-1,3-pentanedione (Hphen5f) Synthesize with embodiment one;
[0089] 2. Dimer of iridium (dfppy) 4 Ir 2 Cl 2 The synthesis is with embodiment one;
[0090] 3. Iridium complex Ir(dfppy) 2 The synthesis of (phen5f) is the same as in Example 1;
[0091] 4. Double metal complexes (dfppy) 2 Ir(μ-phen5f)EuCl 3 Synthesis
[0092] Ir(dfppy) 2 (phen5f)(0.1mmol) and EuCl 3 ·6H 2 O (0.1 mmol) was dissolved in ethanol, stirred and refluxed for 6 hours. After cooling to room temperature, the solvent was spin-dried, and then recrystallized with ethanol and ether to obtain the pure product (dfppy) 2 Ir(μ-phen5f)EuCl 3 . Elemental analysis values: C, 39.58; H, 2.74; N, 4.56; C 39 h 20 f 9 IrN 4 o 2 ·EuCl 3 ·2CH 3 CH 2 Calcd for OH: C, 40.03; H, 2.50; N, 4.34.
Embodiment 3
[0093] Embodiment three. Double metal complex (ppy) 2 Ir(μ-phen5f)NdD 3 Synthesis
[0094] 1. Tetradentate ligand 4,4,5,5,5-pentafluoro-1-(1',10'-o-phenanthroline-2'-yl)-1,3-pentanedione (Hphen5f) Synthesize with embodiment one;
[0095] 2. Dimer of iridium (ppy) 4 Ir 2 Cl 2 Synthesis
[0096] Referring to the literature (Bull.Chem.Soc.Jpn.1974,47,767), trihydrate iridium trichloride and 2.2 times the equivalent of ligand ppy are mixed in ethylene glycol monoethyl ether and water mixed in a volume ratio of 3:1, Stir and reflux at 120° C. for 24 hours under nitrogen protection, cool to room temperature, filter with suction, wash the solid with ethanol and acetone, and dry in vacuo.
[0097] 3. Iridium complex Ir(ppy) 2 Synthesis of (phen5f)
[0098] Referring to literature (J.Am.Chem.Soc.2001,123,4304), the dimer (ppy) of 0.5mmol iridium 4 Ir 2 Cl 2, 1.1 mmol tetradentate ligand Hphen5f and 5 mmol anhydrous sodium carbonate were mixed in ethylene glycol monoethyl et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com