Process for producing a pair of novel ginsengenin and its compound body, and preparations thereof
A technology of total ginseng saponins and mixtures, applied in the field of medicine, can solve problems such as undiscovered preparation methods and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
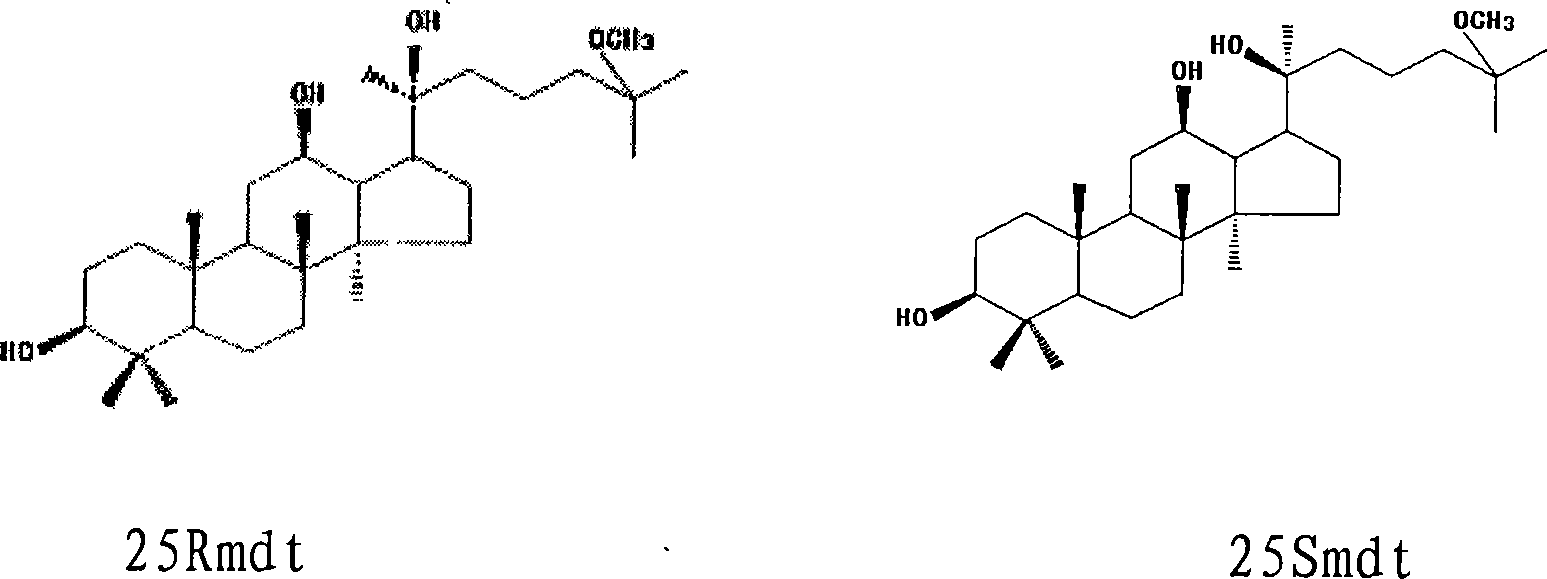
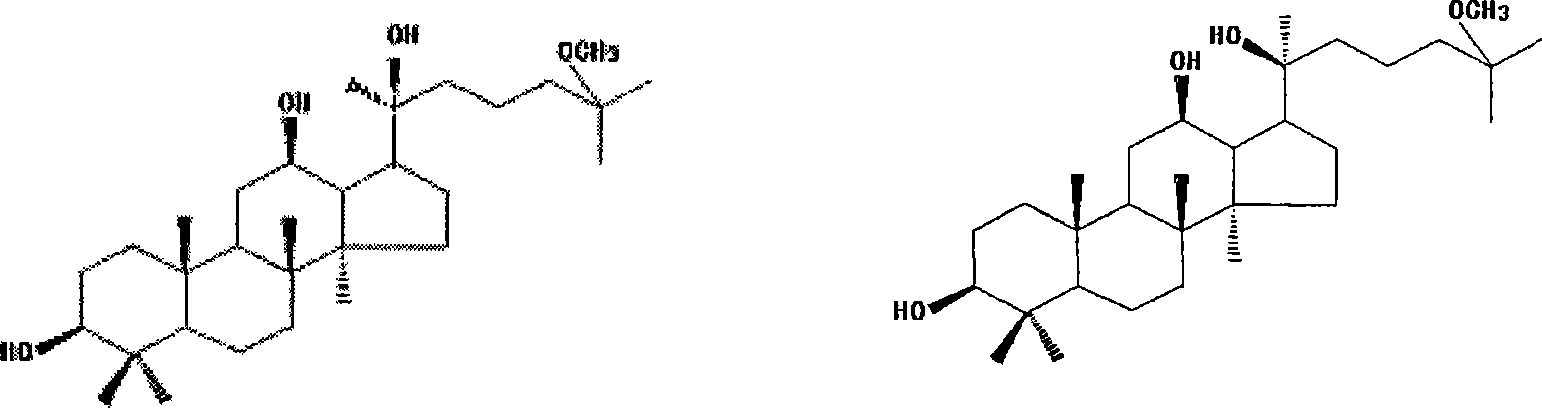
[0026] Embodiment 1: sodium hydroxide hydrolysis method prepares 25Rmdt and 25Smdt and their mixture (racemate)
[0027] Take by weighing 10 g of total saponins of ginseng fruit, dissolve in 1000 ml of sodium hydroxide with a concentration of 2.5 mol / L, and a concentration of 80% in methanol aqueous solution for 24 h, and then use 2.5 mol / L hydrochloric acid to neutralize the reaction solution, recover methanol under reduced pressure, and use The reaction solution was extracted with chloroform, and the chloroform phase was washed with water, dried over anhydrous sodium sulfate, and evaporated to dryness to collect the residue, separated by silica gel column chromatography, and gradient eluted with petroleum ether: ethyl acetate (10:1-1:1) to obtain 86 Fractions, fractions 52-55 were recrystallized from ethyl acetate to obtain 25Rmdt; fractions 56-58 were combined after TLC inspection, and after removing the solvent, ethyl acetate recrystallized to obtain a mixture of 25Rmdt and...
Embodiment 2
[0028] Embodiment 2: hydrochloric acid hydrolysis method prepares 25Rmdt and 25Smdt and their mixture (racemate)
[0029] Weigh 10 g of total saponins from American ginseng leaves, dissolve in 1000 ml of 80% methanol aqueous solution with hydrochloric acid concentration of 2.5 mol / L and sonicate. Ultrasonic conditions: frequency: 50kHz; power: 3KW; time: 30 minutes; hydrolyze at a temperature of 40°C for 12 hours, neutralize the reaction solution with 2.5mol / L sodium hydroxide, recover methanol under reduced pressure, extract the reaction solution with chloroform, and chloroform phase Washed with water, dried over anhydrous sodium sulfate, evaporated to dryness to collect the residue, separated by silica gel column chromatography, chloroform: ethyl acetate (15:1-1:1) gradient elution to obtain 58 fractions, fractions 30-35 were 25Rmdt was obtained after ethyl acetate recrystallization; Fractions 36-38 were combined after TLC inspection, and after removing the solvent, ethyl ac...
Embodiment 3
[0030] Embodiment 3: Acid hydrolysis method prepares 25Rmdt and 25Smdt and their mixture (racemate)
[0031]Weigh 10g of Panax notoginseng total saponins, dissolve it in a mixed solution of 1000ml concentrated hydrochloric acid and 1000ml methanol and sonicate, ultrasonic conditions: frequency: 50kHz; power: 3KW; time: 10 minutes; Sodium oxide neutralized the reaction solution, recovered methanol under reduced pressure, extracted the reaction solution with ether, washed the extract with water, dried over anhydrous sodium sulfate, and evaporated to dryness to collect the residue, separated by silica gel column chromatography, chloroform:methanol (30:1- 5:1) gradient elution yielded 58 fractions, fractions 30-35 were recrystallized from ethyl acetate to obtain 25Rmdt; fractions 36-38 were combined after TLC inspection, and 25Rmdt was obtained after solvent removal and ethyl acetate recrystallization and 25Smdt mixture (racemate); Fractions 38-41 were combined after TLC inspectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com