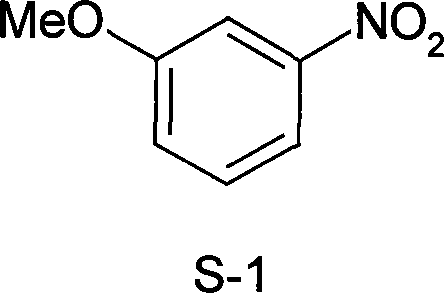
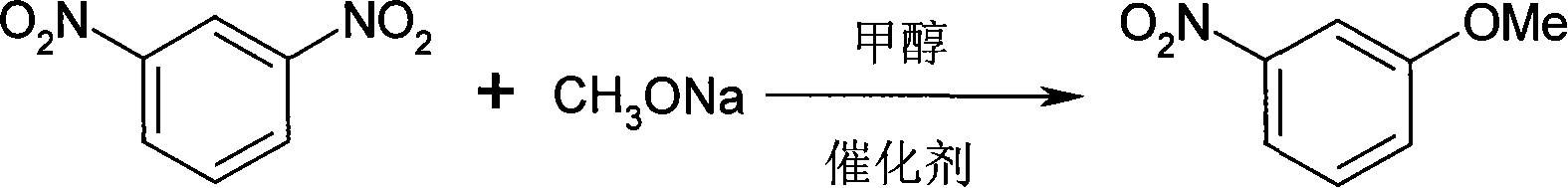
Method for synthesizing 3-methoxy nitrobenzene
A technology of methoxynitrobenzene and synthesis method, which is applied in the field of synthesis of organic compounds, can solve the problems of high price and difficult post-processing, and achieve the effects of reducing costs, increasing output, and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1, a kind of synthetic method of 3-methoxynitrobenzene, carries out following steps successively:
[0015] 1), methoxylation, i.e. the preparation of 3-methoxynitrobenzene crude product:
[0016] 168.0g (1.0mol) m-dinitrobenzene, 135.0g (2.5mol) sodium methylate, 410ml methanol, 0.33g 18-crown-6 ether, 3.36g tetrabutyl bromide were put into an autoclave with stirring and temperature control Ammonium (TBAB), under 1.0 MPa, heated to 150° C. for 10 h to obtain 130.5 g of a dark red solid, which is a crude product of 3-methoxynitrobenzene, and the yield of the crude product was 85.3%.
[0017] 2), recrystallization, i.e. the preparation of 3-methoxynitrobenzene:
[0018] At room temperature, add isopropanol (330 mL) to the above crude 3-methoxynitrobenzene, heat to reflux for 5 h, cool, and vacuum filter. After washing and drying, 121.8 g of white solid was obtained, which was 3-methoxynitrobenzene, and the product yield was 79.6%.
Embodiment 2
[0019] Embodiment 2, a kind of synthetic method of 3-methoxynitrobenzene, carry out following steps successively:
[0020] 1), methoxylation, i.e. the preparation of 3-methoxynitrobenzene crude product:
[0021] 33.6g (0.2mol) m-dinitrobenzene, 21.6g (0.4mol) sodium methoxide, 80ml methanol, 0.02g18-crown-6 ether, 0.17g tetrabutyl bromide were put into the autoclave with stirring and temperature control Ammonium (TBAB), heated at 0.5 MPa to 100° C. for 6.5 h to obtain 26.9 g of a dark red solid, which is a crude product of 3-methoxynitrobenzene, and the yield of the crude product is 87.9%.
[0022] 2), recrystallization, i.e. the preparation of 3-methoxynitrobenzene:
[0023] At room temperature, add n-propanol (100 mL) to the crude 3-methoxynitrobenzene, heat to reflux for 2 h, cool, and vacuum filter. After washing and drying, 25.6 g of white solid was obtained, which was 3-methoxynitrobenzene, and the product yield was 83.6%.
Embodiment 3
[0024] Embodiment 3, a kind of synthetic method of 3-methoxynitrobenzene, carries out following steps successively:
[0025] 1), methoxylation, i.e. the preparation of 3-methoxynitrobenzene crude product:
[0026] 84.0g (0.5mol) m-dinitrobenzene, 34.0g (0.63mol) sodium methylate, 300ml methanol, 0.08g 18-crown-6 ether, 0.84g tetrabutyl bromide were put into the autoclave with stirring and temperature control Ammonium (TBAB), heated at 0.65MPa to 120°C for 5h to obtain 72.7g of a dark red solid, which is a crude product of 3-methoxynitrobenzene, and the yield of the crude product is 95.0%.
[0027] 2), recrystallization, i.e. the preparation of 3-methoxynitrobenzene:
[0028] At room temperature, ethanol (180 mL) was added to the crude 3-methoxynitrobenzene, heated to reflux for 1 h, cooled, and vacuum filtered. After washing and drying, 69.4 g of white solid was obtained, which was 3-methoxynitrobenzene, and the product yield was 90.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com