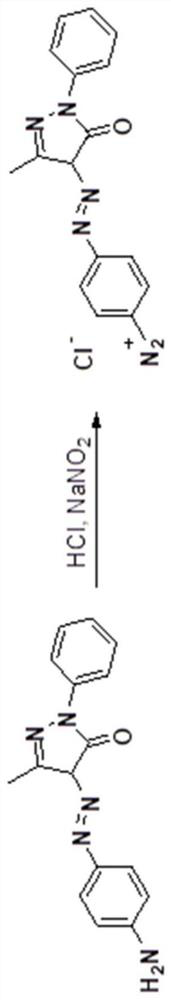
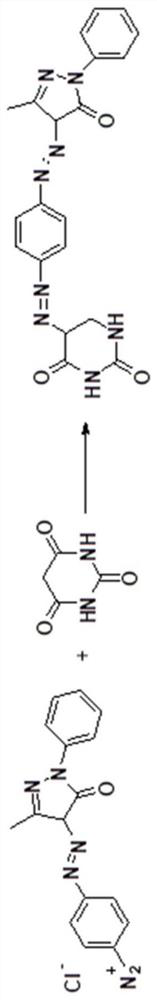
Synthesis method of red bisazo organic pigment
A technology for organic pigments and synthesis methods, applied in the directions of organic dyes, azo dyes, organic chemistry, etc., can solve the problems of waste of resources and difficult post-processing, and achieve the effects of avoiding waste of resources, avoiding post-processing difficulties, and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A kind of synthetic method of red disazo organic pigment, concrete steps are as follows:
[0038] Step 1: The preparation of 3-methyl-4-((4-aminophenyl)diazo)-1-phenyl-1H-pyrazol-5(4H)-one diazonium salt comprises the following steps:
[0039] 1) Diazotize 3-methyl-4-((4-aminophenyl)diazo)-1-phenyl-1H-pyrazol-5(4H)-one, the specific steps are: weigh 293mg (1mmol) 3-methyl-4-((4-aminophenyl) dinitrogen)-1-phenyl-1H-pyrazol-5(4H)-one, 20mL water, 4mL 2mol / L hydrochloric acid, 2mL Add dimethyl sulfoxide into a 100mL single-necked flask, ultrasonically dissolve, and after the reaction solution is cooled to 0°C, quickly add an aqueous solution prepared with 104mg (1.5mmol) sodium nitrite, continue to stir at 0°C for 10min, spot the plate to detect diazo reaction, the Congo red test paper is blue, and the starch potassium iodide test paper is slightly blue. Add an appropriate amount of urea to destroy the excess sodium nitrite, and obtain a red diazonium salt solution (3-met...
Embodiment 2
[0044] A kind of synthetic method of red disazo organic pigment, concrete steps are as follows:
[0045] Step 1: The preparation of 3-methyl-4-((4-aminophenyl)diazo)-1-phenyl-1H-pyrazol-5(4H)-one diazonium salt comprises the following steps:
[0046] 1) Diazotize 3-methyl-4-((4-aminophenyl)diazo)-1-phenyl-1H-pyrazol-5(4H)-one, the specific steps are: weigh 586mg (2mmol) 3-methyl-4-((4-aminophenyl)diazo)-1-phenyl-1H-pyrazol-5(4H)-one, 25mL water, 10mL 5wt% hydrochloric acid, 5mL di Add methyl sulfoxide into a 100mL single-necked flask, stir at 70°C for 1h, cool to 5°C, quickly add 207mg (3mmol) of sodium nitrite in an aqueous solution, continue to stir at 5°C for 10min, spot plate detection of diazotization Reaction, the Congo red test paper is blue, and the starch potassium iodide test paper is slightly blue. Add an appropriate amount of urea to destroy the excess sodium nitrite, and obtain a red diazonium salt solution.
[0047] Step 2: Coupling reaction of diazonium salt a...
Embodiment 3
[0054] Compared with Example 1, most of them are the same, except that in this example, 4mL 2mol / L hydrochloric acid is changed to 1.5mL 2mol / L hydrochloric acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com