Water relaxation-based sensors
A sensor and relaxation time technology, used in instruments, scientific instruments, magnetic variable measurement, etc., can solve the problem of nanoparticles not responding to the weak magnetic field of hand-held magnets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0191] Embodiment 1 Glu-CLIO nano-conversion
[0192] overview
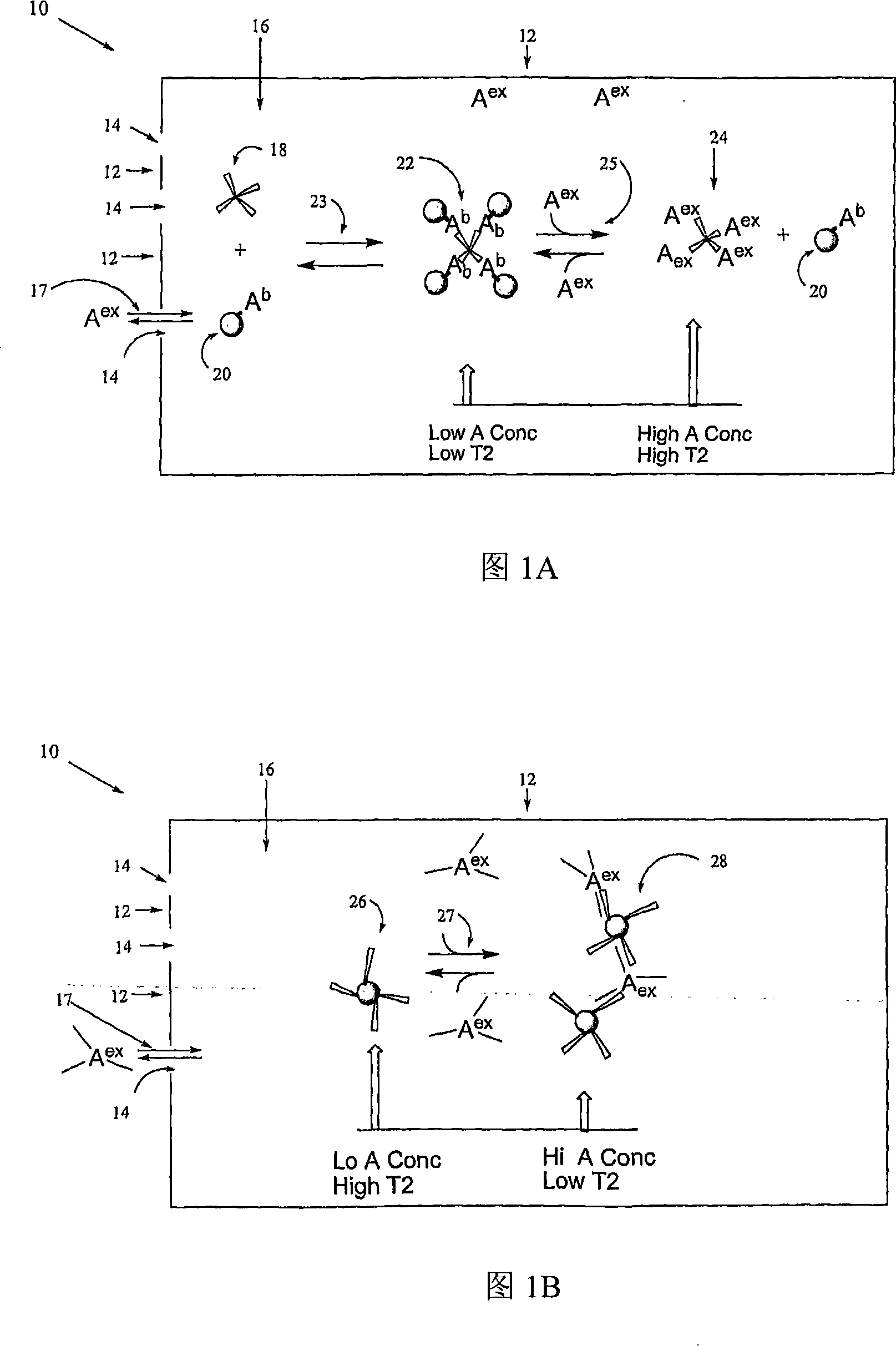
[0193] To demonstrate the water relaxation sensor, we designed a model machine for monitoring physiological concentrations of glucose. We used (con)conavalin A as binding protein and synthetic glucose-functionalized magnetic nanoparticles (Glu-CLIO). Concanavalin A (ConA) is a tetravalent lectin known to react with glucose. Some sensors were prepared with wall shells with a pore size of 3 kDa. In general, the wall shell of the sensor holds the Glu-CLIO nanoparticles and ConA, while allowing glucose to freely pass in and out of the sensor.
Embodiment 1A
[0194] Preparation of Example 1A.Glu-CLIO
[0195] MION-47 and amino-CLIO (25-35 nm) were prepared as described elsewhere. D-glucose, D-(+)-glucosamine hydrochloride, succinic anhydride, (con)concanavalin A (ConA) and Sephadex G-25 were from Sigma Aldrich. 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) and sulfo-N-hydroxysuccinimide (sulfo-NHS) were from Pierce (Rockford, IL). In order to synthesize glucose-functionalized nanoparticles (Glu-CLIO), the NH 2 -CLIO was transformed into carboxyl-functionalized nanoparticles, which were then coupled to 2-amino-glucose using water-soluble carbodiimide. To obtain carboxy-functionalized CLIO, add 2.0 mg succinic anhydride to 200 uL NH 2 -CLIO (10mgFe / mL, 42NH 2per 2064 Fe) with 300uL (0.1M) NaHCO 3 buffer, pH 8.5. The mixture was incubated for two hours at room temperature and eluted with MES buffer (0.5M NaCl, 0.05MMES), pH 6.0, using a Sephadex G-25 column to remove succinic acid. To conjugate 2-amino-gluc...
Embodiment 1B
[0196] Example 1B. Analysis of Glu-CLIO-ConA-Glucose Tubes
[0197] Relaxation times were obtained using a Minispec stress relaxometer (Bruker) at 0.47T, 40°C.
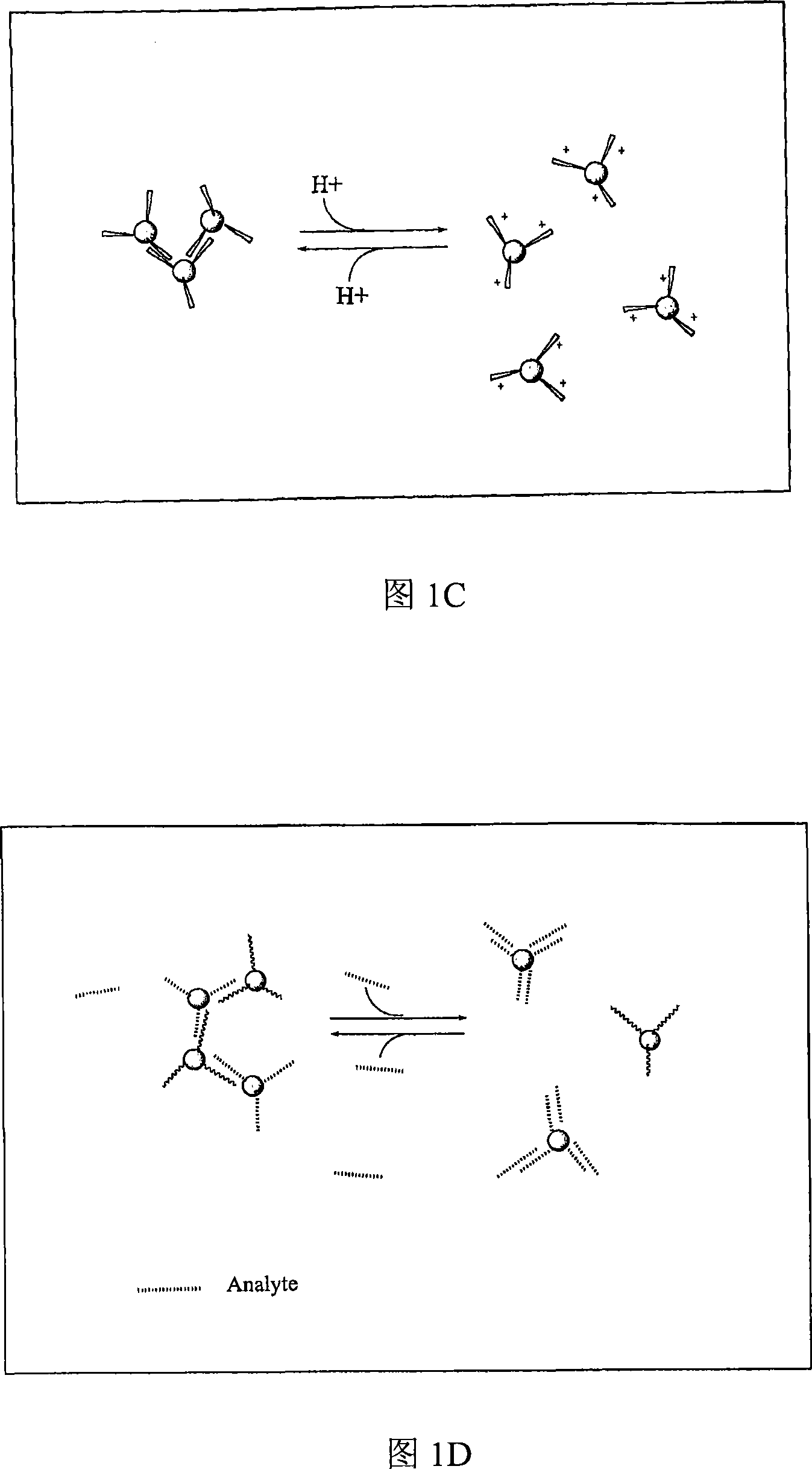
[0198] To demonstrate the interaction of Glu-CLIO, ConA and glucose, experiments were performed directly in NMR tubes (without semipermeable wall enclosures), 10ugFe / mL, 800ug / mL ConA. All experiments were performed with lmM CaCl 2 and 1mM MgCl 2 performed in PBS. Transverse relaxation times (T2's) were measured with a stress relaxometer Bruker Minispec(R) NMS 120 at 0.47T and 40°C. Size was determined with a Zetasizer 1000(R) (Malvern Instruments, Marlboro, MA) in the above buffer with Glu-CLIO at 20 ug Fe / mL, followed by addition of ConA to 1 mg / mL and 1.5 mg / mL glucose. These concentrations of ions, buffer, ConA and Glu-CLIO were used in all experiments.
[0199] As shown in Figure 3A, addition of ConA to Glu-CLIO decreased T2, which reached a plateau after about 50 min, while there was no change for amino-CLI...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com