Process for synthesizing parthenolide derivative and uses thereof
A technology of parthenolide and a synthesis method, which can be applied in directions such as drug combinations, nervous system diseases, non-central analgesics, etc., can solve problems such as reducing production costs, and achieve the effects of reducing production costs, high safety, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
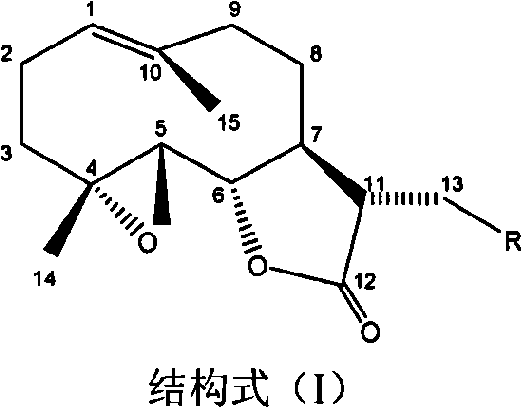
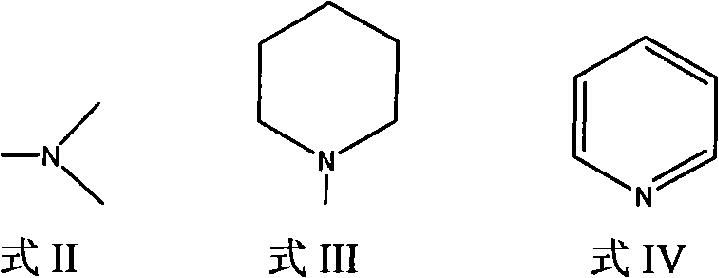
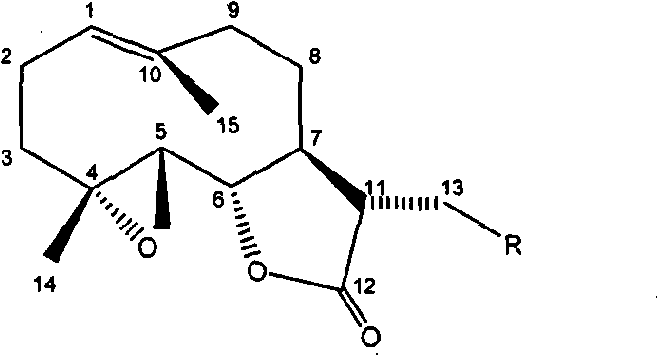
[0029] Preparation of 11,13-dihydro-13-dimethylamino parthenolide
[0030] Add 200mg (0.8mmol) of parthenolide, 180mg (4mmol) of dimethylammonia, and 476mg (7mmol) of sodium ethoxide into 80mL of ethanol, stir at room temperature for 24 hours, make it fully react, evaporate the solvent under reduced pressure, and the residue After silica gel column chromatography, chloroform-methanol 50:1-1:1 gradient elution, TLC or HPLC detection, collect fractions containing 11,13-dihydro-13-dimethylaminoparthenolide, and finally obtain 216 mg of product (yield 92%), compound purity 98.51%. Compound melting point: 142.8-1143.7°C; 1 H-NMR (500MHz, CDCl3): δ5.24(1H, d), 3.82(1H, t), 2.76(2H, m), 2.64(1H, dd), 2.53-2.29(3H, m), 2.27( 6H, s), 2.20-1.95 (5H, m), 1.68 (3H, s), 1.35 (3H, s), 1.30-1.16 (1H, m);
[0031] 13 C-NMR (500MHz, CDCl 3 ): δ175.9, 134.8, 124.2, 82.1, 66.8, 61.9, 58.1, 47.9, 46.5, 46.1, 40.8, 36.2, 30.3, 24.5, 17.8, 16.8; EI-MS: M / Z293 (M + ); UV(MeOH) λmax(logε): 210n...
Embodiment 2
[0033] Preparation of 11,13-dihydro-13-piperidinyl parthenolide
[0034] Add 200mg (0.8mmol) of parthenolide, 272mg (3.2mmol) of piperidine, and 476mg (7mmol) of sodium ethoxide into 80mL of ethanol, stir at room temperature for 24 hours, make it fully react, evaporate the solvent under reduced pressure, and the residue Silica gel column chromatography, chloroform-methanol 50:1-1:1 gradient elution, TLC or HPLC detection, collected fractions containing 11,13-dihydro-13-piperidinyl parthenolide, and finally obtained 233 mg of product (yield 87%), compound purity 99.26%.
Embodiment 3
[0036] Preparation of 11,13-dihydro-13-pyridyl parthenolide
[0037] Add 200mg (0.8mmol) of parthenolide, 0.53mL (4.8mmol) of pyridine, and 476mg (7mmol) of sodium ethoxide into 80mL of ethanol, stir at room temperature for 24 hours, and make it fully react. Silica gel column chromatography, chloroform-methanol 50: 1-1: 1 gradient elution, TLC or HPLC detects, collects the fraction containing 11,13-dihydro-13-pyridyl parthenolide, and finally obtains 213 mg of product ( Yield 81%), compound purity 98.37%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com