Method for important midbody of strontium ranelate
A technology of ethyl thiophenecarboxylate and compounds, which is applied in the field of key intermediates for the preparation of anti-osteoporosis drug strontium ranelate, can solve the problems that the compound of formula I is not mentioned, and achieve the improvement of purity, the solution of crystallization problems, and the ease of reaction controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
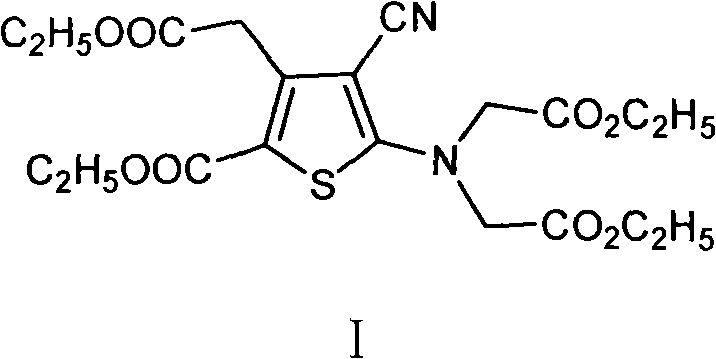
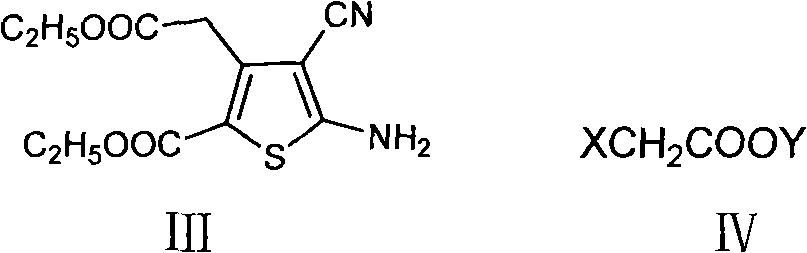
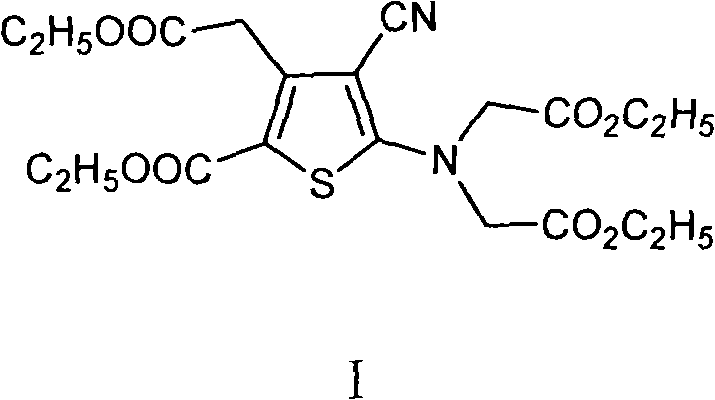
[0024] Add 10g 5-amino-4-cyano-3-(2-ethoxyl-2-oxoethyl)-2-thiophenecarboxylic acid ethyl ester, 14.7g anhydrous potassium carbonate and 120ml butanone in 250ml there-necked flask , stir to dissolve. Heat to reflux for 20 minutes. Under reflux conditions, 41.4 g of ethyl bromoacetate was added dropwise. After dropping, the reaction was detected by TLC. After the reaction was completed, suction filtration was performed, and the filtrate was concentrated under reduced pressure to obtain 40.7 g of dark brown oil. Freeze, suction filter, dry to constant weight, obtain 13.7g light brown solid powder, namely 5-[bis(2-ethoxyl-2-oxoethyl)amino]-4-cyano-3-(2 -Ethoxy-2-oxoethyl)-2-thiophenecarboxylic acid ethyl ester, the yield is 90%. HPLC purity 97%.
Embodiment 2
[0026] Add 10g 5-amino-4-cyano-3-(2-methoxy-2-oxoethyl)-2-thiophenecarboxylic acid ethyl ester, 13.5g anhydrous sodium carbonate and 120ml acetone in 250ml there-necked flask, Stir to dissolve. Heat to reflux for 20 minutes. Under reflux conditions, 41.4 g of ethyl bromoacetate was added dropwise. After dropping, the reaction was detected by TLC. After the reaction was completed, suction filtration was performed, and the filtrate was concentrated under reduced pressure to obtain 40.7 g of dark brown oil. Freeze, filter with suction, and dry to constant weight to obtain 5-[bis(2-ethoxy-2-oxoethyl)amino]-4-cyano-3-(2-ethoxy-2-oxo Ethyl)-2-thiophenecarboxylic acid ethyl ester, the yield is 65%. HPLC purity 93%.
Embodiment 3
[0028] Add 10g 5-amino-4-cyano-3-(2-ethoxy-2-oxoethyl)-2-thiophenecarboxylic acid ethyl ester, 14.7g anhydrous potassium carbonate and 90ml N in the 250ml there-necked flask, N-dimethylformamide, stirred to dissolve. After heating to 60-70°C, 41.4 g of ethyl bromoacetate was added dropwise. After dropping, the reaction was detected by TLC. After the reaction was completed, suction filtration was performed, and the filtrate was concentrated under reduced pressure to obtain 40.7 g of dark brown oil. Freeze, filter with suction, and dry to constant weight to obtain 5-[bis(2-ethoxy-2-oxoethyl)amino]-4-cyano-3-(2-ethoxy-2-oxo Ethyl)-2-thiophenecarboxylic acid ethyl ester, the yield was 73%. HPLC purity 92.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com