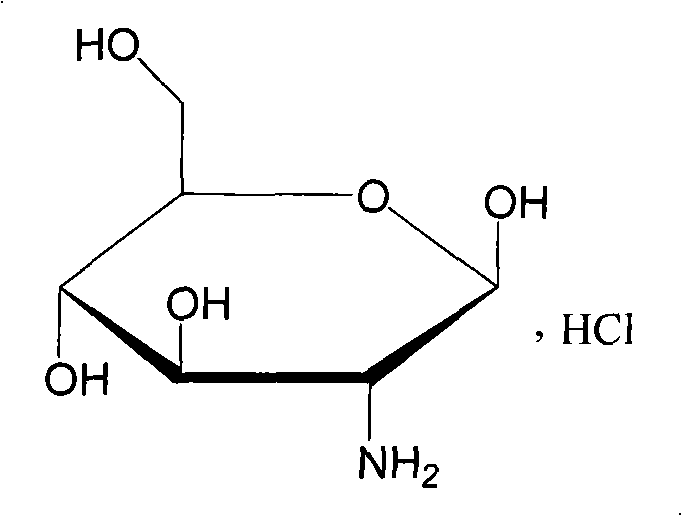
Analytical method of glucosamine
A technology of glucosamine and glucosamine sulfate, applied in the field of analytical chemistry, can solve the problems of low specificity, inaccuracy and convenience, etc., and achieve the effects of strong specificity, easy operation and fast analysis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0027] Experimental Example 1-Selection of High Performance Liquid Chromatography Conditions
[0028] Instruments and reagents: Japan Shimadzu LC-10A high-performance liquid chromatograph and ultraviolet detector are used. The control and recording of the chromatographic separation system are completed by the Wemaron chromatographic workstation. Glucosamine hydrochloride reference substance was purchased from China National Institute for the Control of Pharmaceutical and Biological Products; glucosamine hydrochloride samples were purchased from Zhejiang Chengyi Pharmaceutical Co., Ltd.; It was ultrapure water, and other reagents were of analytical grade.
[0029] Column: Ultimate from Welch Materials TM XB-NH2, 5μm, 4.6×250mm; column temperature: 30°C.
[0030] Detection wavelength: Glucosamine hydrochloride has no characteristic absorption peak, and the ultraviolet absorption is in the terminal ultraviolet region, so 195nm is selected as the detection wavelength.
[0031] ...
experiment example 2
[0034] Experimental Example 2-Selection of High Performance Liquid Chromatography Conditions
[0035] Instruments and reagents: Japan Shimadzu LC-10A high-performance liquid chromatograph and ultraviolet detector are used. The control and recording of the chromatographic separation system are completed by the Wemaron chromatographic workstation. Glucosamine hydrochloride reference substance was purchased from China National Institute for the Control of Pharmaceutical and Biological Products; glucosamine hydrochloride samples were purchased from Zhejiang Chengyi Pharmaceutical Co., Ltd.; It was ultrapure water, and other reagents were of analytical grade.
[0036] Column: Ultimate from Welch Materials TM XB-NH2, 5μm, 4.6×250mm; column temperature: 30°C.
[0037] Detection wavelength: Glucosamine hydrochloride has no characteristic absorption peak, and the ultraviolet absorption is in the terminal ultraviolet region, so 195nm is selected as the detection wavelength.
[0038] ...
experiment example 3
[0042] Experimental Example 3-Selection of High Performance Liquid Chromatography Conditions
[0043]Instruments and reagents: Japan Shimadzu LC-10A high-performance liquid chromatograph and ultraviolet detector are used. The control and recording of the chromatographic separation system are completed by the Wemaron chromatographic workstation. Glucosamine hydrochloride reference substance was purchased from China National Institute for the Control of Pharmaceutical and Biological Products; glucosamine hydrochloride samples were purchased from Zhejiang Chengyi Pharmaceutical Co., Ltd.; It was ultrapure water, and other reagents were of analytical grade.
[0044] Column: Ultimate from Welch Materials TM XB-NH2, 5μm, 4.6×250mm; column temperature: 30°C.
[0045] Detection wavelength: Glucosamine hydrochloride has no characteristic absorption peak, and the ultraviolet absorption is in the terminal ultraviolet region, so 195nm is selected as the detection wavelength.
[0046] M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com