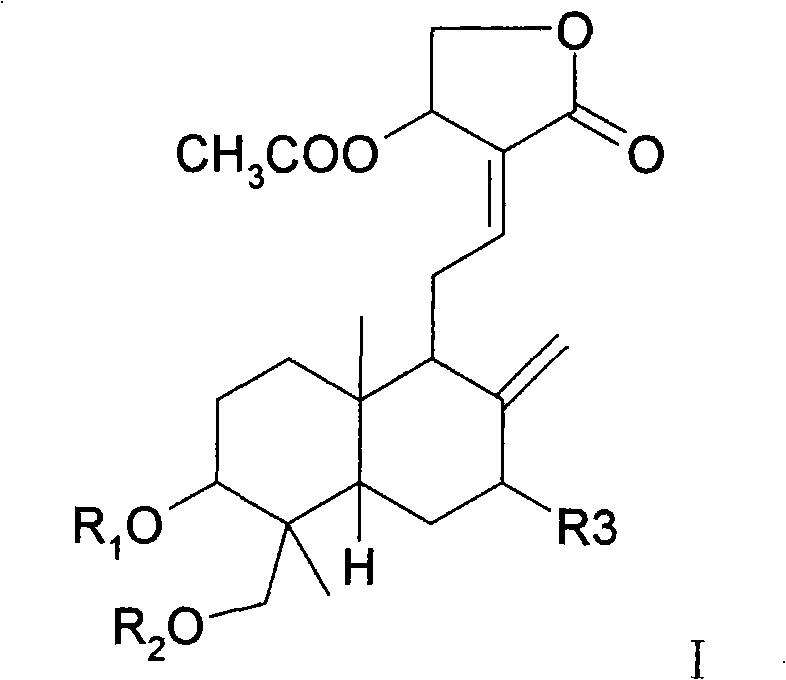
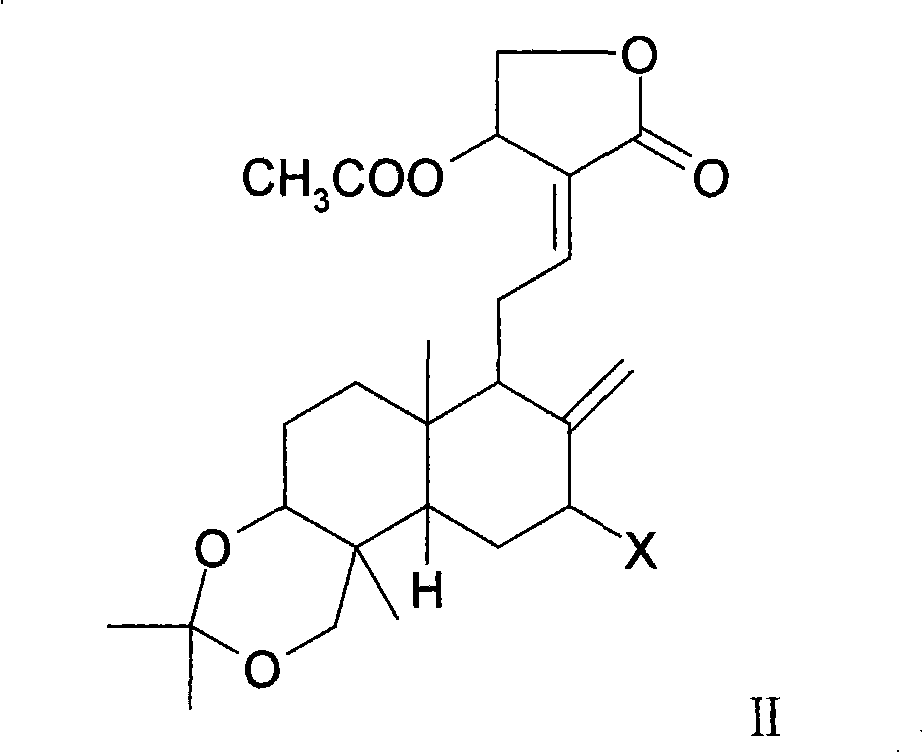
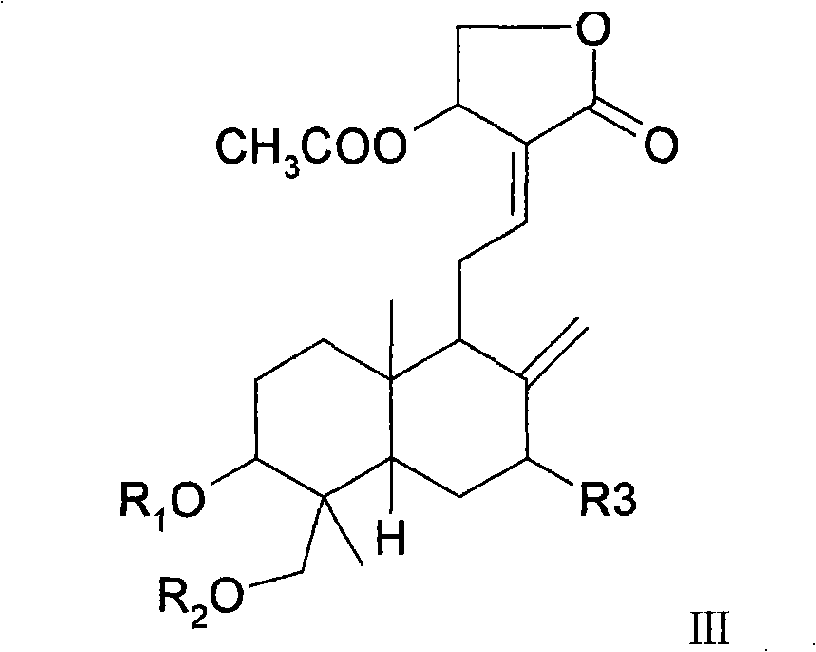
Medicinal composition containing andrographolide derivatives and preparation method thereof and application
A technology of andrographolide and composition, which is applied in the field of pharmaceutical composition containing andrographolide derivatives and its preparation and application, which can solve the problems of undiscovered pharmacy research reports and achieve the effect of stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 13,1
[0036] Preparation Example 13, Preparation of 19-isopropylidene-14-acetylandrographolide (abbreviated as AP5)
[0037] Dissolve 5g of andrographolide (abbreviated as AP), 20mL of 2,2-dimethoxypropane and a catalytic amount of p-toluenesulfonic acid in 100mL of acetone, heat and reflux overnight, extract the reaction mixture with ethyl acetate, and the organic layer is sequentially washed with saturated NaHCO 3 Wash with saturated brine, anhydrous Na 2 SO 4 After drying, concentration, and silica gel column chromatography, 3,19-isopropylidene andrographolide (abbreviated as AP2) was obtained as a white solid. 1g AP2 dissolved in 10mL Ac 2 O, heated to reflux for 2 hours, the reaction mixture was extracted with ethyl acetate, the organic layer was successively water, saturated NaHCO 3 Wash with saturated brine, anhydrous Na 2 SO 4 dry. AP5 was obtained as a white solid by silica gel column chromatography. The molecular formula is: C 25 h 36 o 6 .
[0038]
[0039]...
preparation example 23,14
[0046] Preparation Example 23, 14, the preparation of 19-triacetylandrographolide (abbreviated as AP10)
[0047] 1g of andrographolide was dissolved in 10mL of Ac 2 O, heated to reflux for 2 hours, the reaction mixture was extracted with ethyl acetate, the organic layer was successively water, saturated NaHCO 3 and saturated brine, anhydrous MgSO 4 dry. AP10 was obtained as a white solid by silica gel column chromatography. The molecular formula is: C 26 h 36 o 8 .
[0048]
[0049] Compound confirmation data are as follows:
[0050] 3454, 3080, 2954, 1731, 1683, 1375, 1250, 1191, 1075, 1026, 895, 606.
[0051] 1 H (400MHz, CDCl 3 ):δ
[0052] 7.00 (td, J=6.84, 1.48Hz, 1H, H-12), 5.92 (d, J=5.96Hz, 1H, H-14), 4.90 (bs, 1H, H-17a), 4.60 (dd, J =11.68, 4.26Hz, 1H, H-15a), 4.55(dd, J=11.24, 6.08Hz, 1H, H-15b), 4.53(bs, 1H, H-17b), 4.36(d, J=11.80Hz , 1H, H-19a), 4.25(dd, J=11.24, 1.80Hz, 1H, H-3), 4.12(d, J=11.80Hz, 1H, H-19b), 2.52~2.37(m, 3H) , 2.12(s, 3H), 2...
preparation example 314,19
[0056] Preparation Example 314, the preparation of 19-diacetylandrographolide (AP12 for short) and 14-acetylandrographolide (AP13 for short)
[0057] 5g of andrographolide was added to 30mL of acetic acid, stirred and heated to 80°C, and reacted overnight. The reaction mixture was extracted with ethyl acetate, the organic layer was washed with water and saturated brine successively, and anhydrous MgSO 4 dry. AP12 and AP13 were obtained by silica gel column chromatography. AP13 is a white solid. The molecular formula is: C 22 h 32 o 6 .
[0058]
[0059] Compound confirmation data are as follows:
[0060] 3436, 2927, 1738, 1641, 1384, 1233, 1077, 1024, 723, 621
[0061] 1 H (400MHz, Acetone): δ
[0062] 6.88 (td, J=6.92, 1.68Hz, 1H, H-12), 6.03 (d, J=6.04Hz, 1H, H-14), 4.87 (d, J=1.20Hz, 1H, H-17a), 4.59(d, J=1.08Hz, 1H, H-17b), 4.59~4.57(m, 1H, OH-19), 4.58(dd, J=10.94, 5.94Hz, 1H, H-15a), 4.26(dd , J=11.00, 1.76Hz, 1H, H-15b), 4.11 (d, J=10.88Hz, 1H, H-19a), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com