Quality evaluation process of inactivate effect of methylen blue photochemical virus and quality-controlling products thereof
A virus inactivation and chemical inactivation technology, applied in biochemical equipment and methods, using vectors to introduce foreign genetic material, DNA/RNA fragments, etc., can solve the problems of cumbersome operation, long experiment cycle, and high price, and achieve operational Simple, short experiment cycle, good accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
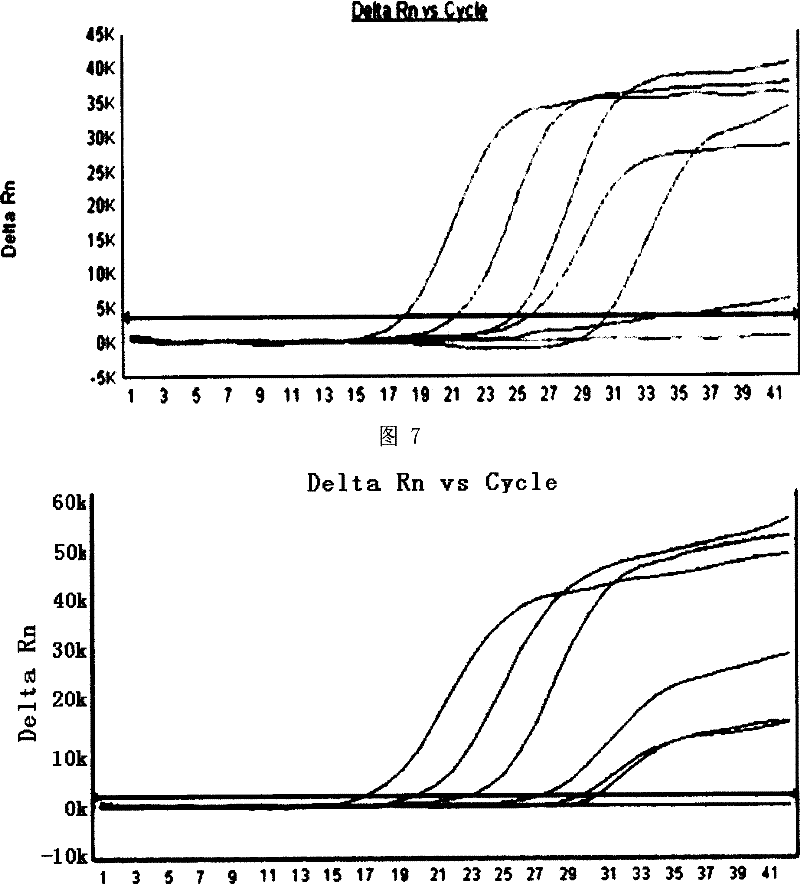
[0094] Embodiment 1 Methylene blue photochemical inactivation has destructive effect on HCV virus nucleic acid
[0095] Experimental steps:
[0096] 1. Cloning establishment:
[0097] According to the HCV1b sequence (AY460204) in GenBank, PCR primers covering HCV 5'-NCR and core region fragments were designed using Gene runner software. The upstream primer sequence is: 5'-CGGAATTCCGACCATAGATACTCCCCT-3' (SEQID NO: 7), and the downstream primer sequence It is: 5'-CGGGATCC CGGAAGATAGAGAAAGAGCAACCG-3' (SEQ ID NO: 8), the length of the amplified fragment is 844bp, and the primers are synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd. After extracting HCV RNA in plasma (Shanghai Blood Center) of blood donors who were positive for both anti-HCV and HCV RNA, Trizol (Gibco BRL) reagent was used, and RNA PCR KIT (Takara Company) was used for reverse transcription and PCR reactions. The reaction parameters were: reverse transcription at 42°C for 30 minutes, pre-...
Embodiment 2
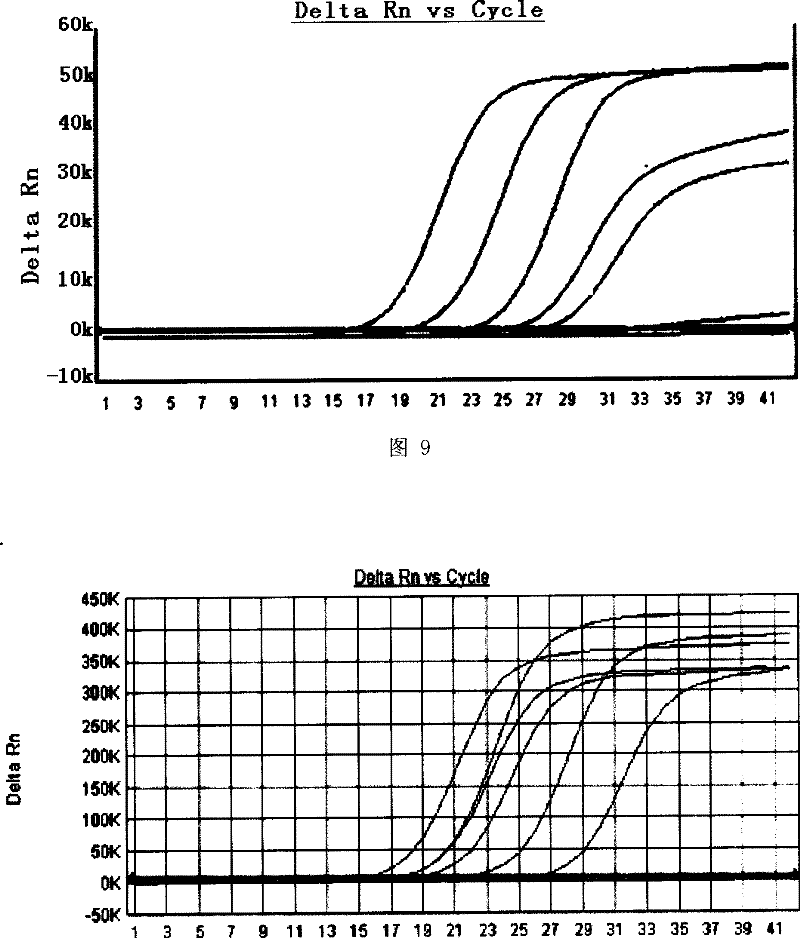
[0110] Example 2 Methylene blue photochemical inactivation has a destructive effect on SV virus nucleic acid
[0111] Experimental steps:
[0112] 1. Preparation of experimental SV and preparation of virus-containing plasma:
[0113] (1) Preparation of SV for experiment
[0114] SV was provided by the Institute of Virology, Chinese Academy of Preventive Medicine, and BHK cells were used as hosts for 3 generations. Collect the supernatant and measure the virus titer >8.51gTCID 50 / ml, aliquoted, stored at -80°C, and used for later use.
[0115] (2) Preparation of virus-containing plasma:
[0116] In the PVC plastic bag containing 100ml anticoagulant plasma, inoculate the virus according to the capacity ratio of plasma: virus solution=9:1, and mix well.
[0117] 2. Virus inactivation experiment:
[0118] In the above-mentioned virus-containing plasma / solution, add methylene blue (MB) so that the final concentrations are 0.5 μmol / L, 1.0 μmol / L, and 1.5 μmol / L, and then irradi...
Embodiment 3
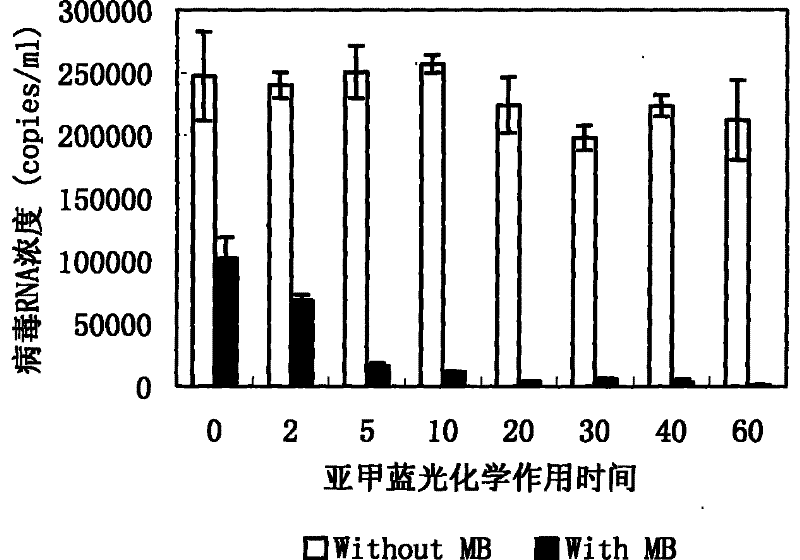
[0129] Example 3 Methylene blue photochemical inactivation has destructive effect on VSV virus nucleic acid
[0130] Experimental steps:
[0131] 1. Preparation of VSV for experiment and preparation of virus-containing plasma:
[0132] (1) Preparation of VSV for experiment
[0133] VSV was provided by the Institute of Virology, Chinese Academy of Preventive Medicine, and was passed on for 3 generations with Vero cells as the host. Collect the supernatant and measure the virus titer >8.51gTCID 50 / ml, aliquoted, stored at -80°C, and used for later use.
[0134] (2) Preparation of virus-containing plasma:
[0135] In the PVC plastic bag containing 100ml anticoagulant plasma, inoculate the virus according to the capacity ratio of plasma: virus solution=9:1, and mix well.
[0136] 2. Virus inactivation experiment:
[0137] In the above-mentioned virus-containing plasma / solution, add methylene blue (MB) so that the final concentrations are 0.5 μmol / L, 1.0 μmol / L, and 1.5 μmol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com