Quality evaluation process of inactivate effect of methylen blue photochemical virus and quality-controlling products thereof
A technology for virus inactivation and quality evaluation, applied in biochemical equipment and methods, using vectors to introduce foreign genetic material, DNA/RNA fragments, etc., can solve the problems of long experimental period, cumbersome operation, expensive price, etc. Short, easy to operate, good accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] 1. Cloning establishment:
[0080] According to the HCV1b type sequence (AY460204) in GenBank, PCR primers covering HCV 5'-NCR and core region fragments were designed using Gene runner software. The sequence is: 5'-CGGGATCC CGGAAGATAGAGAAAGAGCAACCG-3' (SEQ ID NO: 4), the length of the amplified fragment is 844bp, and the primers are synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd. After extracting HCV RNA in plasma (Shanghai Blood Center) of blood donors who were positive for both anti-HCV and HCV RNA, Trizol (Gibco BRL) reagent was used, and RNA PCR KIT (Takara Company) was used for reverse transcription and PCR reactions. The reaction parameters were: reverse transcription at 42°C for 30 minutes, pre-denaturation at 94°C for 2 minutes, denaturation at 94°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 1 minute, with 30 cycles of denaturation, annealing, and extension. After the PCR product was purified, it was c...
Embodiment 2
[0097] 1. Preparation of experimental SV and preparation of virus-containing plasma:
[0098] (1) Preparation of SV for experiment
[0099] SV was provided by the Institute of Virology, Chinese Academy of Preventive Medicine, and BHK cells were used as hosts for 3 generations. Collect the supernatant and measure the virus titer>8.5lgTCID 50 / ml, aliquoted, stored at -80°C, and used for later use.
[0100] (2) Preparation of virus-containing plasma:
[0101] In the PVC plastic bag containing 100ml anticoagulant plasma, inoculate the virus according to the capacity ratio of plasma: virus solution=9:1, and mix well.
[0102] 2. Virus inactivation experiment:
[0103] In the above-mentioned virus-containing plasma / solution, add methylene blue (MB) so that the final concentrations are 0.5 μmol / L, 1.0 μmol / L, and 1.5 μmol / L, and then irradiate with 30000 Lux visible light, and the irradiation time is 0′( not illuminated), 30', 60'.
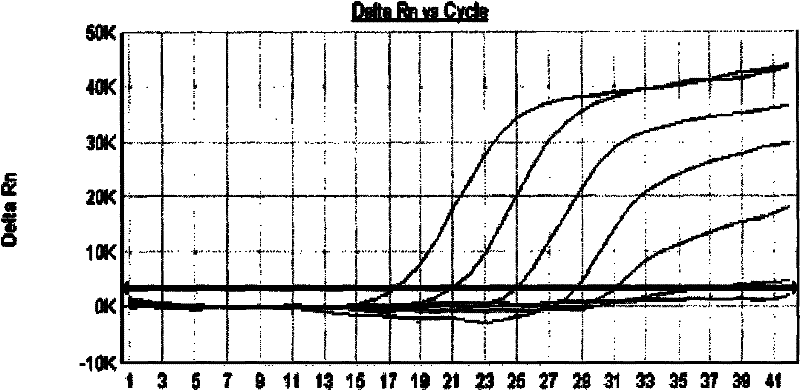
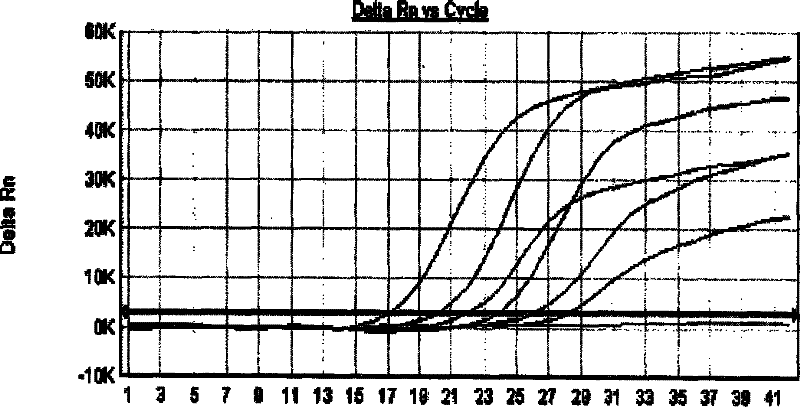
[0104] 3. Viral nucleic acid detection (qua...
Embodiment 3
[0114] 1. The inactivated product of step 2 in Example 2 is sampled respectively, inoculated with BHK cells, and measured by cytopathic method ("Medical Experimental Virology", People's Military Medical Publishing House (first edition) P102-109 Du Ping). Infectious titer (TCID 50 / ml), the results are as follows:
[0115] Infectious inactivation results of SV at different concentrations and different contact times
[0116]
[0117] *Indicates that the method used has not detected the virus titer
[0118] The results confirmed that after inactivation, the virus infectivity disappeared.
[0119] 2. Result analysis: It can be seen from Example 2 that under MB-P treatment, SV virus RNA was degraded, and this experiment proved that after MB-P treatment, SV virus infectivity disappeared, thus confirming the viral nucleic acid There is a certain correlation between the degradation of the virus and the disappearance of the infectivity of the virus.
[0120] Example 4 Preparatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com