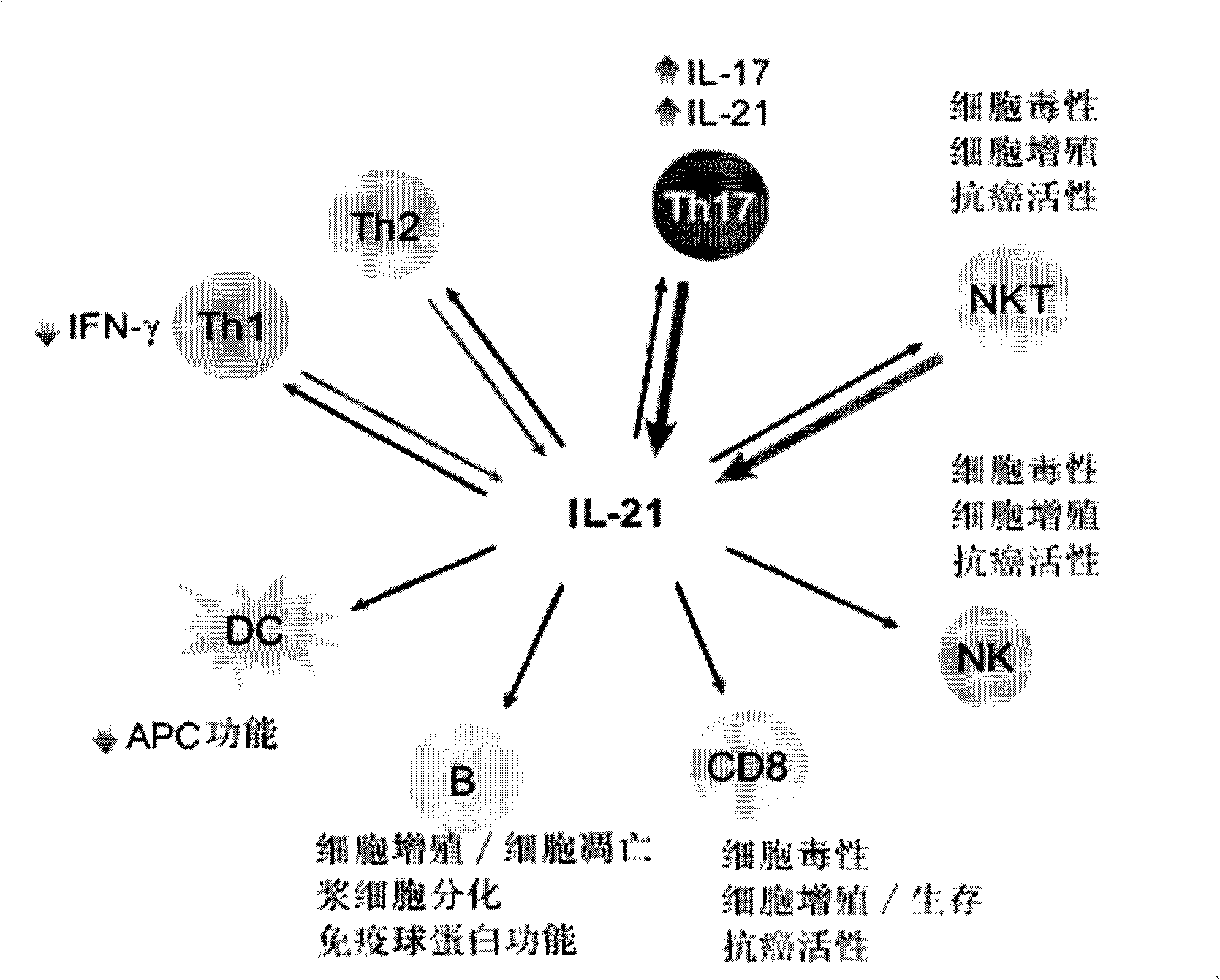
Human
A fully human antibody and antibody technology, applied in the field of bioengineering, can solve problems such as the application limitation of mouse antibody drugs, and achieve the effects of high affinity, convenient transformation and low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Screening of fully human anti-IL-21 single chain antibody
[0056] 1.1 Preparation of TG1 and HB2151 with sex cilia
[0057] The phage was infected by binding to the cilium of Escherichia coli, and TG1 and HB2151 were streaked from the plate in the glycerol cryopreservation tube, inoculated on the M9 medium plate, and cultured upside down at 37°C for 36 hours. Store at 4°C and use within 1 week.
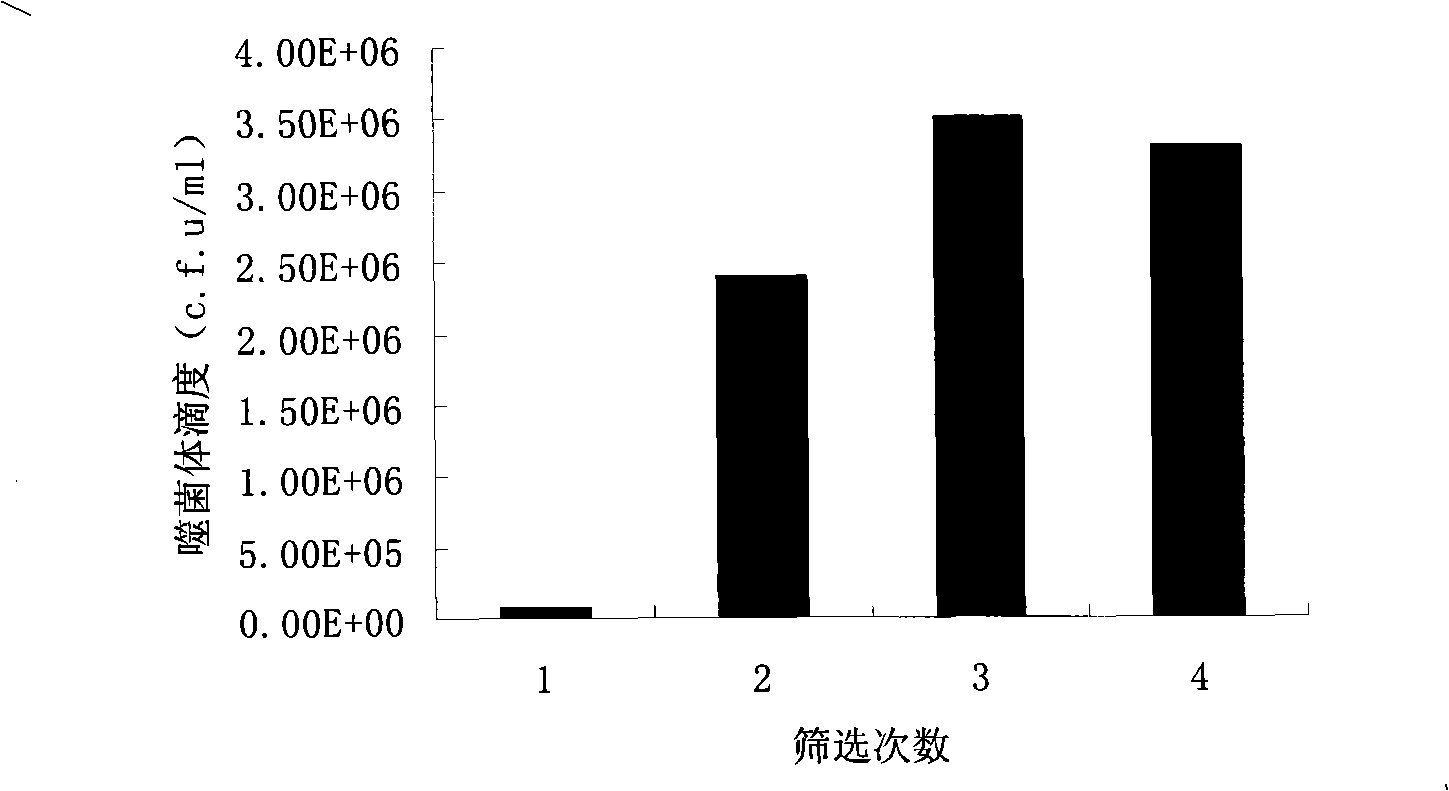
[0058] 1.2 Determination of wild-type M13 phage titer
[0059] (1) Pick the TG1 single clone on the M9 plate, inoculate it in 2×TY medium, and culture it overnight (8-16 hours) at 37° C. with shaking.
[0060] (2) Take the TG1 cultured overnight, inoculate it in 2×TY medium at 1% (v / v), and cultivate it with shaking at 37°C until the logarithmic phase (OD 600 ≈0.5).
[0061] (3) Lay the lower plate TYE (1.5% agar powder).
[0062] (4) The wild-type phage frozen in glycerol was serially diluted to 10 times by 100 times. -12 10 μl of wild-type phage in each dilut...
Embodiment 2
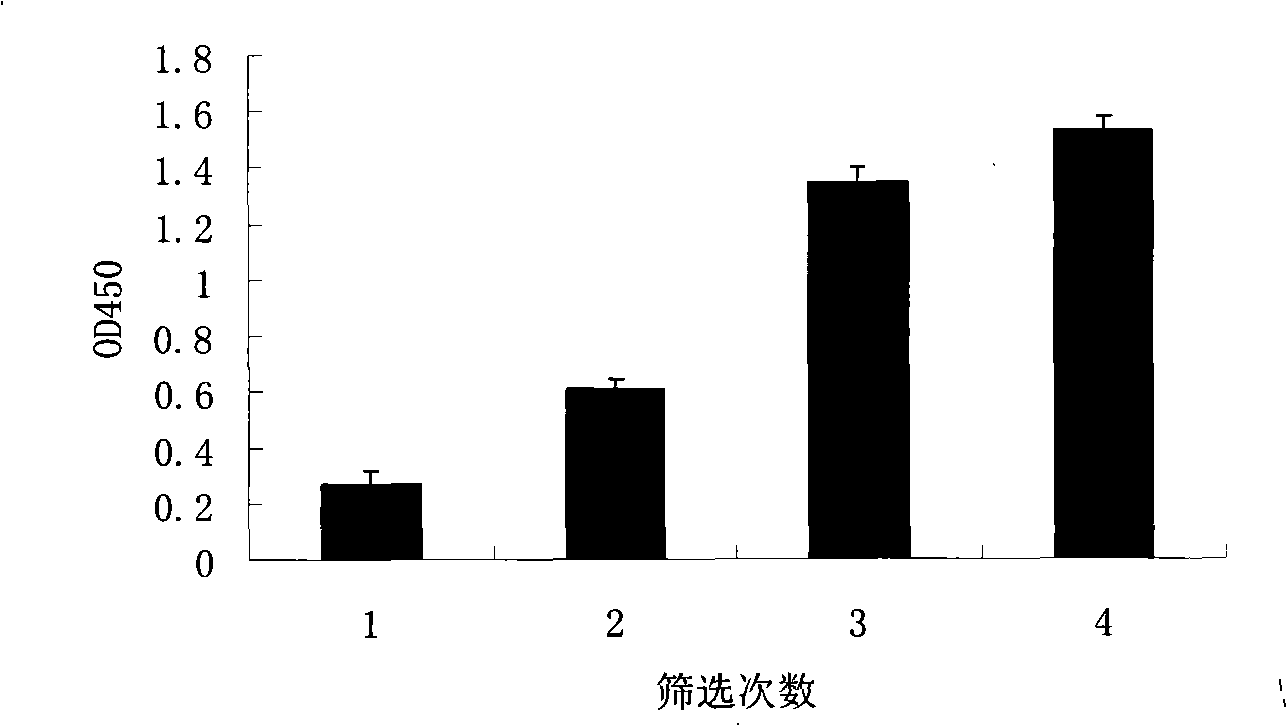
[0118] Embodiment 2 screening identification
[0119] 2.1 Polyclonal phage ELISA
[0120] (1) Dilute human IL-21 to 10 μg / ml with PBS, add 100 μl to each well, and coat overnight at room temperature.
[0121] (2) The plate was washed 3 times with PBS, and blocked with 200 μl 3% BSA (PBS configuration) for 2 hours.
[0122] (3) Wash the plate 3 times with PBS. After each round of screening, 10 μl of phage obtained by PEG / NaCl precipitation was taken, diluted to 100 μl with 3% BSA-PBS, and added to a microtiter plate.
[0123] (4) Incubate at room temperature for 90 min, wash 3 times with PBS containing 0.05% Tween20, and then wash 3 times with PBS.
[0124] (5) Add 100 μl goat anti-M13 polyclonal antibody diluted 1:1000 with 3% BSA to each well, and incubate at room temperature for 90 minutes. Wash 3 times with PBS containing 0.05% Tween20, and then 3 times with PBS.
[0125] (6) Add 100 μl of HRP-rabbit anti-goat IgG polyclonal antibody diluted 1:5000 with 3% BSA, and inc...
Embodiment 3
[0139] Example 3 Antibody Soluble Expression and Affinity Determination
[0140] 3.1 Antibody Soluble Expression and Purification
[0141] (1) Take 1 μl of the bacteria in the wells whose monoclonal ELISA value is more than 3 times higher than that of the blank, inoculate into 1ml 2×TY medium, and culture on a shaker at 37°C until OD 600 ≈0.5.
[0142] (2) Add helper phage VCS-M13 and infect for 30 minutes at 37° C., the infection ratio is the number of bacteria / number of helper phage=1 / 20.
[0143] (3) The bacterial cells were collected by centrifugation at 3300 rpm for 10 min, and the bacterial cell pellet was resuspended in 1 ml of 2×TY medium (containing 100 μg / ml ampicillin and 25 μg / ml kanamycin). Shake overnight at 30°C.
[0144] (4) Centrifuge at 12,000 rpm for 10 minutes to remove bacteria, and infect 10 ml of HB215 in logarithmic phase at 37° C. for 130 minutes with the supernatant obtained by centrifugation.
[0145] (5) Centrifuge the infected HB2151 at 3300rpm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com