Preparation method for direct methanoic acid fuel cell palladium-on-carbon nano-catalyst
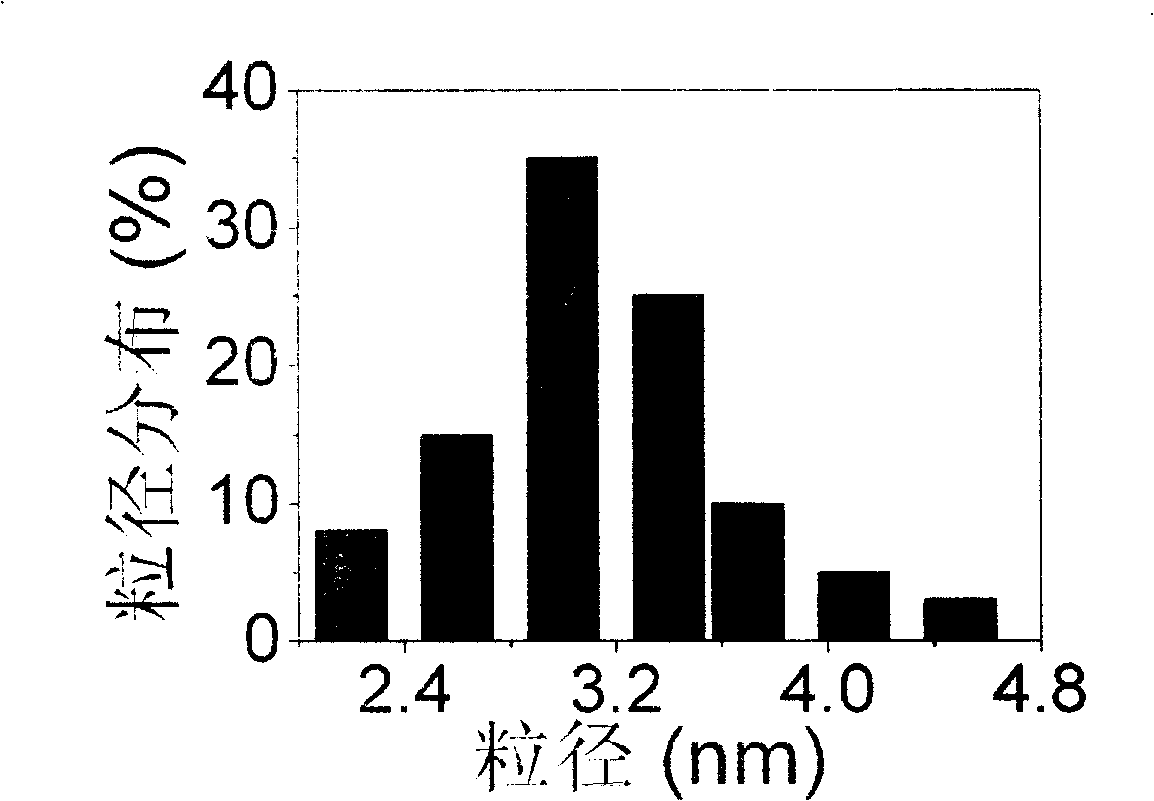
A formic acid fuel cell and nano-catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, battery electrodes, etc., can solve problems such as not suitable for mass production, high cost, complicated preparation process, etc., and achieve great application potential, The effect of small particle size and uniform particle distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Disperse 47.3 mg of activated carbon in 100 ml of a mixed solution of water and isopropanol with a volume ratio of 1:1, disperse and ultrasonically vibrate for 2 hours to obtain a suspension of activated carbon, add 25 ml of PdCl dissolved in hydrochloric acid 2 aqueous solution, hydrochloric acid and PdCl in the precursor aqueous solution 2 The molar concentrations are 7.2mM and 6mM respectively; then add 25ml 2mM sodium tungstate aqueous solution and stir for two hours; then add 50ml NaBH 4 aqueous solution for reduction, where NaBH 4 The number of moles is 8 times the number of moles of palladium, stirred for 8 hours, the resulting mixture was filtered and washed with water, and vacuum-dried at 80° C. for 10 hours to obtain a direct formic acid fuel cell anode carbon-supported palladium catalyst.
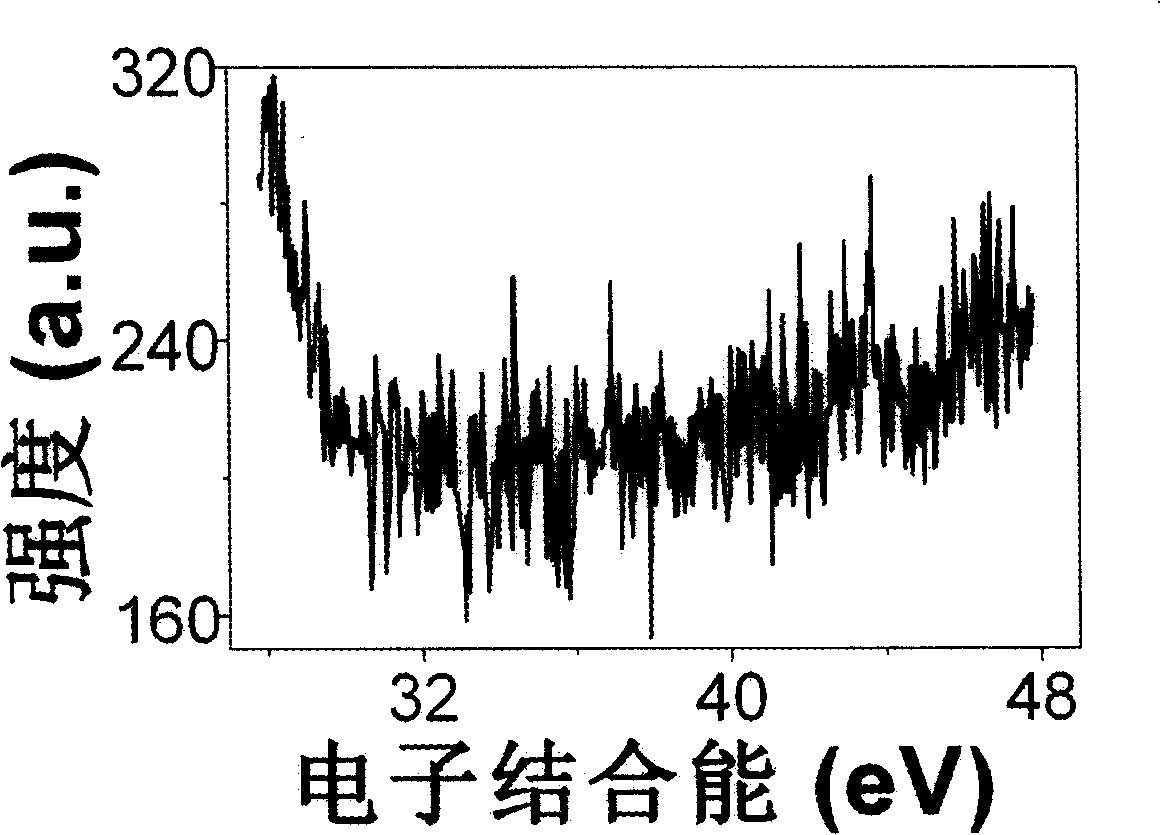
[0014] From figure 1 It can be seen in the photoelectron spectrum of , in the range of 30 to 45eV, there is no W 4f The characteristic peaks indicate that the W species...
Embodiment 2
[0016] Other conditions are the same as in Example 1, except that the molar concentration of the sodium tungstate aqueous solution is changed to 1 mM. The catalyst was not used (WO 3 ) n ·xH 2 Comparison of the electrochemical performance of formic acid oxidation with catalysts prepared by the common impregnation method of O colloidal sources. the result shows: image 3 The former peak current for the catalytic oxidation of formic acid shown in b, is image 3 The latter shown in e is 1.80 times the peak current for the catalytic oxidation of formic acid; Figure 4 The stability of the former to the catalytic oxidation of formic acid shown by c is better than Figure 4 The stability of the latter towards the catalytic oxidation of formic acid shown in d.
Embodiment 3
[0018] Other conditions are the same as in Example 1, only the molar concentration of the sodium tungstate aqueous solution is changed to 4mM. The catalyst was not used (WO 3 ) n ·xH 2 Comparison of the electrochemical performance of formic acid oxidation with catalysts prepared by the common impregnation method of O colloidal sources. the result shows: image 3 The former peak current for the catalytic oxidation of formic acid shown in c, is image 3 The latter shown in e is 1.75 times the peak current for the catalytic oxidation of formic acid; Figure 4 The stability of the former to the catalytic oxidation of formic acid shown by b is better than that of Figure 4 The stability of the latter towards the catalytic oxidation of formic acid shown in d.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com