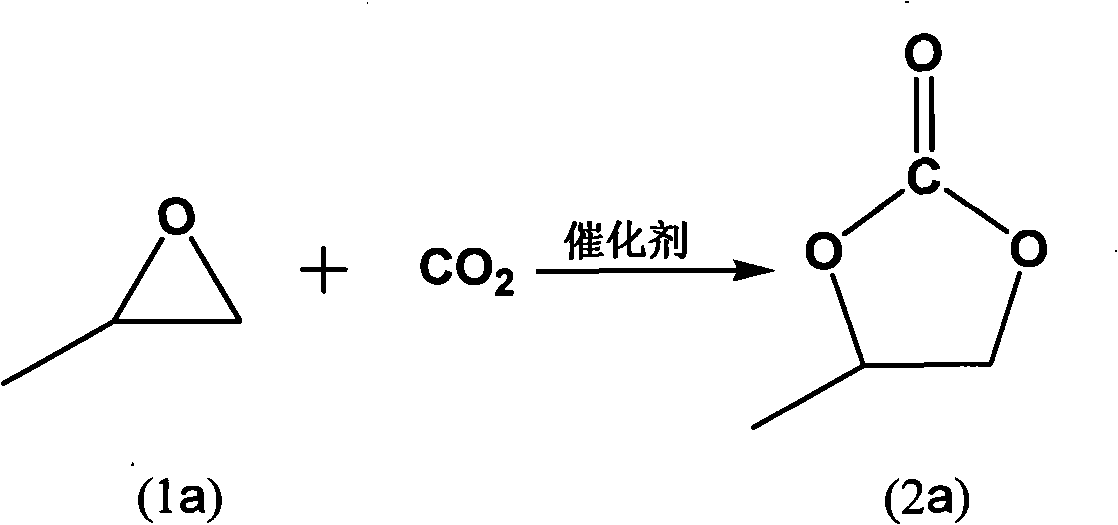
Process for synthesizing cyclic carbonate with catalysis of solid carried ion liquid catalyst
A cyclic carbonate, ionic liquid technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc. lower problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
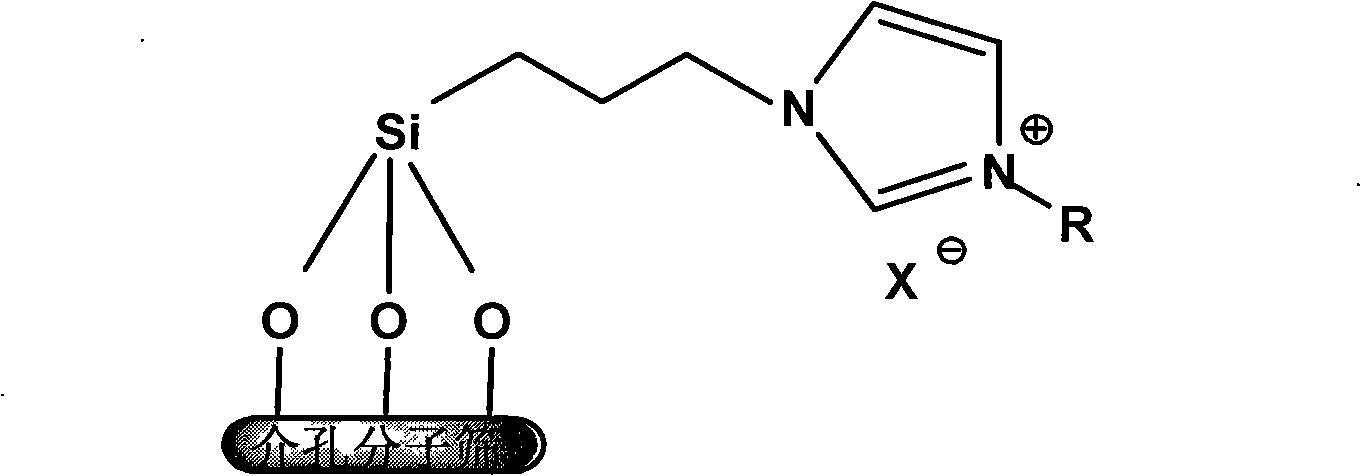
[0012] The modified MCM-41 reference [Nie Chunfa, Suo Jiquan. Molecular Catalysis, 2004, 18(1):61] was prepared. 4g of modified MCM-41 and 2g of imidazole were refluxed in xylene for 4h, filtered, and the solid was washed with absolute ethanol and dried under vacuum at 60°C, then 5g of imidazole-modified MCM-41 was taken and 3g of bromoethane was added. Reflux in xylene at 120°C for 24h, filter, and dry to obtain ethylimidazolium bromide ionic liquid supported by MCM-41.
Embodiment 2
[0014] The modified MCM-41 was prepared in the same manner as in Example 1. 4g of the modified MCM-41 and 3g of imidazole were refluxed in xylene for 4h, filtered, the solid was washed with absolute ethanol, and dried in vacuum at 60°C, and 5g of imidazole was taken. Add 3.5g of bromobutane, reflux in xylene at 120°C for 24h, filter and dry to obtain MCM-41-supported butylimidazolium bromide ionic liquid.
Embodiment 3
[0016] The modified MCM-41 was prepared in the same manner as in Example 1. 4g of the modified MCM-41 and 2g of imidazole were refluxed in xylene for 4h, filtered, the solid was washed with absolute ethanol, and dried under vacuum at 60°C, and then 5g of imidazole was taken. Add 4g of bromohexane, reflux in xylene at 120°C for 24h, filter and dry to obtain the hexylimidazolium bromide ionic liquid supported by MCM-41.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com