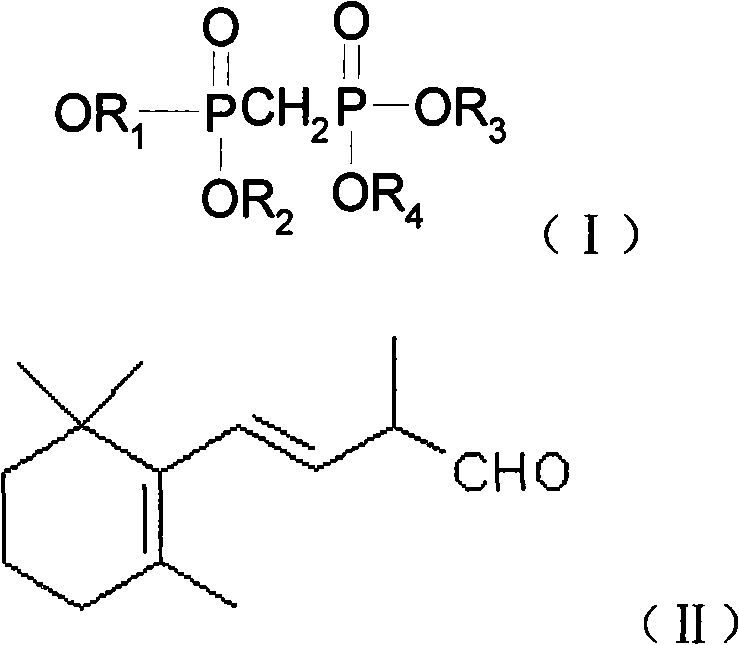
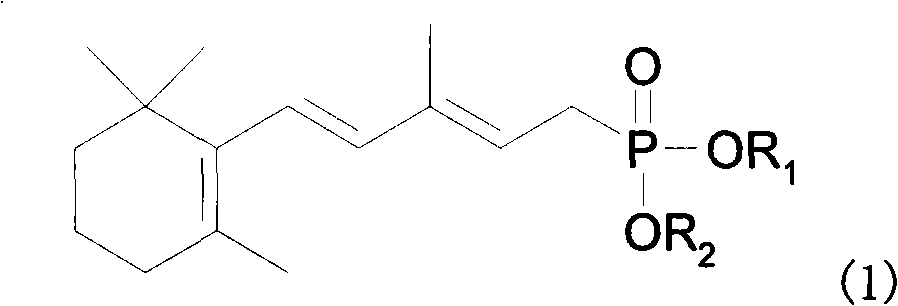
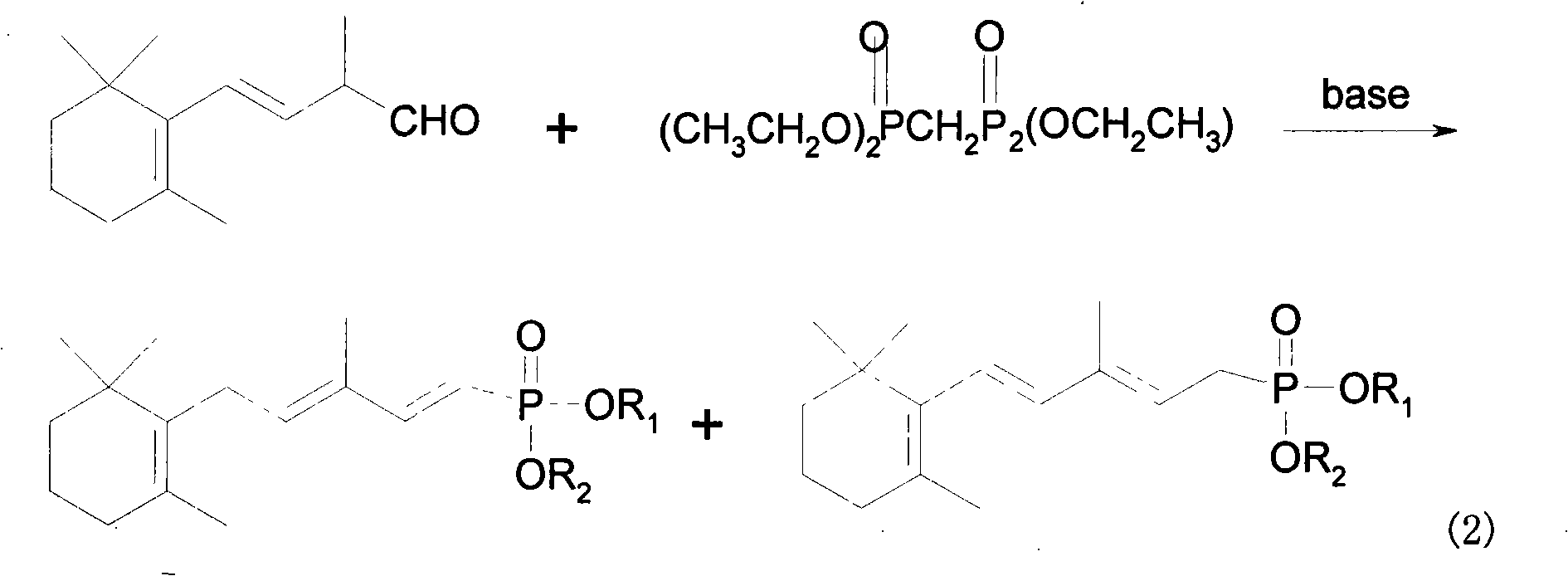
Method for preparing 3-methyl-5-(2,6,6-trimethyl-1-cyclohexene-1-base)-2, 4-pentadiene-dialkyl phosphoric ester
A technology of dialkyl pentadienyl phosphonate and tetraalkyl methylene diphosphate, which is applied in the field of 3-methyl-5--2, can solve the problem that the conversion rate is only 76%, and the yield of the target product is reduced. The efficiency and difficulty of separating the target product increase, and achieve the effect of benefiting industrial production, reducing energy consumption and difficulty of production operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0018] 1.76 millimoles (508 milligrams) of tetraethyl methylene diphosphate and 5 milliliters of a mixed solvent of tetrahydrofuran and toluene with a volume ratio of 1:3 were mixed and added to the reactor, and 0.01 g of 2-hydroxyl-4- Methoxybenzophenone, adjust the temperature to be 10°C, then add 1.76 mmoles of sodium methylate, and drop 1.01 mmoles (208 mg) of C after 20 minutes of reaction 14 Aldehydes, after continuing to react for 10 minutes, add water equivalent to 20ml times the volume of the organic solvent to wash, dry, and distill under reduced pressure to obtain 320mg of the final product, 3-methyl-5-(2,6,6-trimethyl-1-cyclo Hexen-1-yl)-2,4-pentadienyl diethyl diacetate, the yield was 93%, detected by gas chromatography,
[0019] The final product is detected by gas chromatography under the following chromatographic conditions:
[0020] Capillary column SE-54 (30m*0.25mm*0.25um)
[0021] Carrier gas is N 2 Purity greater than 99.999%
[0022] Column temperatur...
example 2
[0028] 1.76 millimoles (605 milligrams) of tetraisopropyl methylene diphosphate and 7 milliliters of a mixed solvent of tetrahydrofuran and toluene with a volume ratio of 1:4 were mixed and added to the reactor, and 0.01 grams of 2-hydroxyl-4- Octyloxybenzophenone, adjust the temperature to be 10°C, then add 1.76 mmoles of sodium ethylate, and drop 1.41 mmoles (290 mg) of C after 20 minutes of reaction 14 Aldehyde, after continuing to react for 15 minutes, add water equivalent to 25ml times the volume of the organic solvent to wash, dry, and distill under reduced pressure to obtain 440 mg of the final product, with a yield of 89%. Detected by gas chromatography, 3-methyl-5-(2 , 6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienyl diisopropyl phosphonate accounted for 85.8%. Diisopropyl 3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-1,3-pentadienylphosphonate accounted for only 3.2%.
example 3
[0030] 1.76 millimoles (605 milligrams) of tetraisopropyl methylene diphosphate and 7 milliliters of a mixed solvent of tetrahydrofuran and toluene with a volume ratio of 1:4 were mixed and added to the reactor, and 0.01 grams of 2-hydroxyl-4- Octyloxybenzophenone, adjust the temperature to be 20°C, then add 1.76 mmoles of sodium ethylate, and drop 1.41 mmoles (290 mg) of C after 20 minutes of reaction 14 Aldehydes, after continuing to react for 15 minutes, add water equivalent to 25ml times the volume of the organic solvent to wash, dry, and distill under reduced pressure to obtain 430mg of the final product, with a yield of 86.9%. Detected by gas chromatography, 3-methyl-5-(2 , 6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienyl diisopropyl phosphonate accounted for 83.4%, 3-methyl-5-(2,6, Diisopropyl 6-trimethyl-1-cyclohexen-1-yl)-1,3-pentadienylphosphonate accounted for only 3.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com