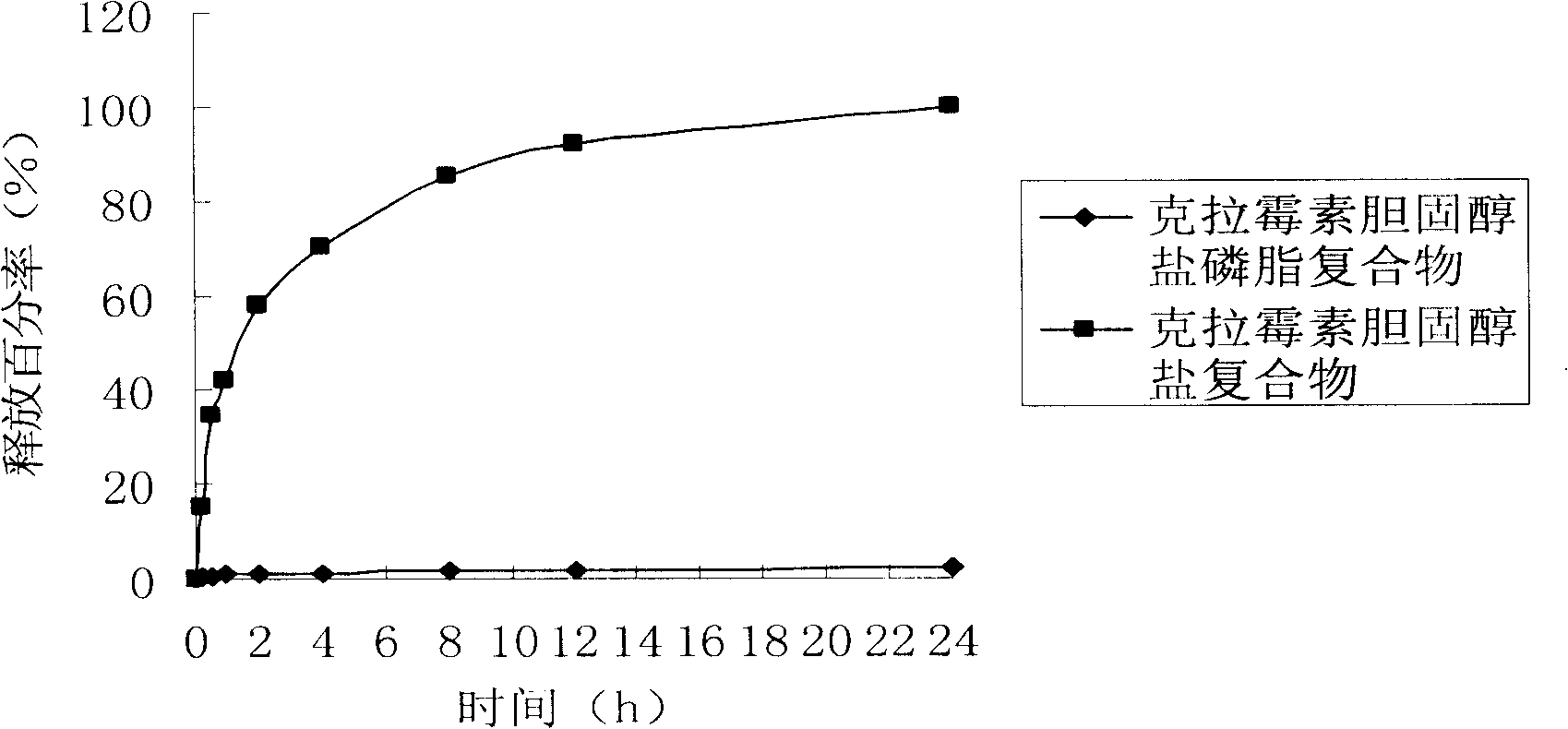
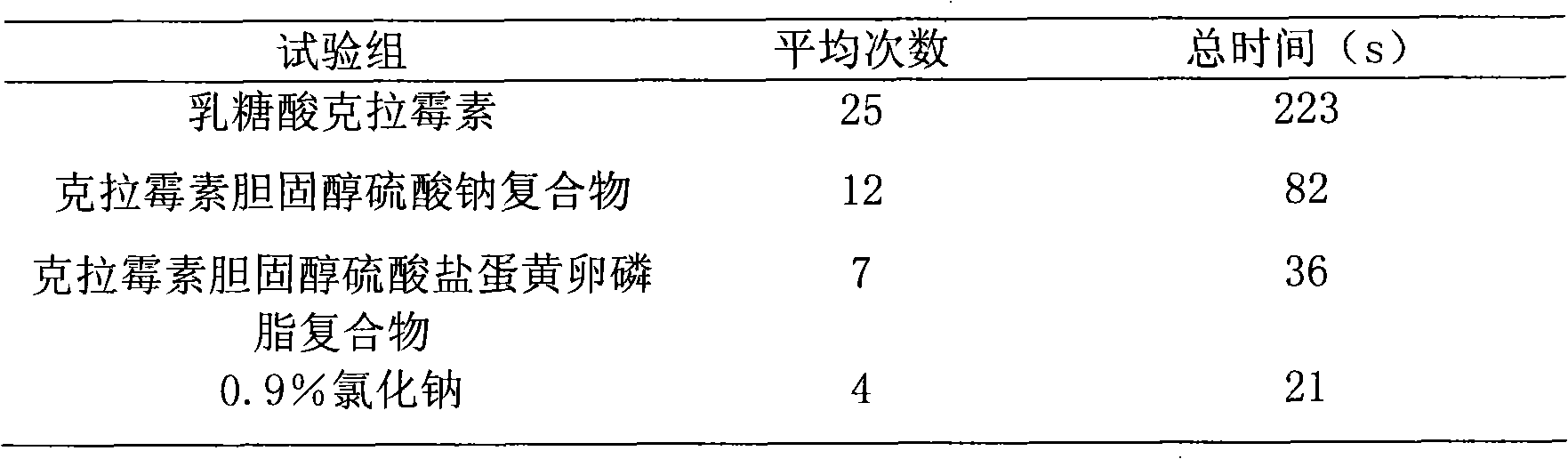
Stable medicament lipid complexes
A lipid complex and drug technology, applied in the field of medicine, can solve problems such as nephrotoxicity and vascular stimulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] formula:
[0016] Sodium cholesteryl sulfate 0.35g (about 0.71mmol)
[0017] Clarithromycin 0.5g (about 0.66mmol)
[0018] Egg yolk lecithin 0.75g (about 0.96mmol)
[0019] Weigh cholesterol sodium sulfate and clarithromycin respectively according to the formula and put them in a 100ml beaker, add 15ml of dimethyl sulfoxide, keep warm in a water bath at 45°C, stir continuously to dissolve; and weigh egg yolk lecithin according to the formula and put them in a 50ml beaker , add 12ml of ethanol, stir continuously to dissolve, keep warm in 45°C water bath. Slowly pour the ethanol solution of phospholipids into the above dimethyl sulfoxide solution, stir gently to mix evenly, keep warm in a water bath at 45°C for later use. Draw the above drug solution with a syringe, and put it into the pH7.2 tris buffer solution at a speed of 5 ml / min until all the drug solution is injected to obtain the clarithromycin lipoplex suspension. During injection, the buffer should be stirre...
Embodiment 2
[0022] formula:
[0023] Sodium cholesteryl sulfate 0.2g (about 0.41mmol)
[0024] Sodium cholesteryl phosphate 0.15g (about 0.291mmol)
[0025] Clarithromycin 0.5g (about 0.67mmol)
[0026] Egg yolk lecithin 0.5g (about 0.64mmol)
[0027] Soy lecithin 0.25g (about 0.32mmol)
[0028]Weigh cholesterol sodium sulfate, cholesterol sodium phosphate, and clarithromycin according to the formula respectively, put them in a 100ml beaker, add 15ml of dimethyl sulfoxide, keep warm in a water bath at 45°C, stir continuously to dissolve; and weigh egg yolk lecithin according to the formula Put soybean lecithin and soybean lecithin in a 50ml beaker, add 12ml ethanol, stir continuously to dissolve, and keep warm in a water bath at 45°C. Slowly pour the ethanol solution of phospholipids into the above dimethyl sulfoxide solution, stir gently to mix evenly, keep warm in a water bath at 45°C for later use. Draw the above drug solution with a syringe, and inject / inject it into the pH7.2 bu...
Embodiment 3
[0031] formula:
[0032] Amphotericin B 0.5g (about 0.54mmol)
[0033] Sodium Cholesterol Phosphate 0.3g (about 0.58mmol)
[0034] Egg yolk lecithin 0.5g (about 0.64mmol)
[0035] Weigh amphotericin B and cholesteryl sodium phosphate according to the formula, put them in a 100ml beaker, add 15ml of dimethyl sulfoxide, keep warm in a water bath at 45°C, and stir continuously to dissolve; in addition, weigh egg yolk lecithin according to the formula and dissolve it in 10ml In slightly hot ethanol, slowly add the dimethyl sulfoxide solution of the above drug, and stir to mix evenly. Keep warm in a 45°C water bath for later use. Use a syringe to draw the above drug solution and slowly inject it into the pH7.2 buffer solution. This process should be stirred slowly to accelerate the diffusion of the organic phase. After the drug solution is injected, the amphotericin B lipoplex suspension is obtained, the organic solvent and a small amount of free drug are removed, and concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com