Process for production of bisphenol-A
A bisphenol and phenol technology, which is applied in the field of preparing bisphenol A, can solve the problems of ion exchange resin catalyst activity reduction, etc., and achieve the effects of inhibiting activity reduction, preventing deterioration, and long-term stable production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] In the method for preparing bisphenol A of the present invention, each step performed after the above-mentioned reaction step is not particularly limited, and for example, a known method can be adopted. As each step performed after the reaction step, for example, a low boiling point component separation step of separating the reaction mixture obtained in the reaction step into a component containing bisphenol A and a low boiling point component containing unreacted acetone; The components of bisphenol A and phenol are precipitated in the form of crystals of the adduct of bisphenol A and phenol to obtain the crystallization step of the slurry; the slurry obtained in the crystallization step is separated into crystals and mother liquor, and the adduct of bisphenol A and phenol The recovery step of recovering the crystal form of bisphenol A; the crystals of the adduct obtained in the recovery step are melted, distillation, etc. to remove phenol to obtain bisphenol A; after at ...
Embodiment 1~3
[0032] Examples 1 to 3, Comparative Examples 1 to 2:
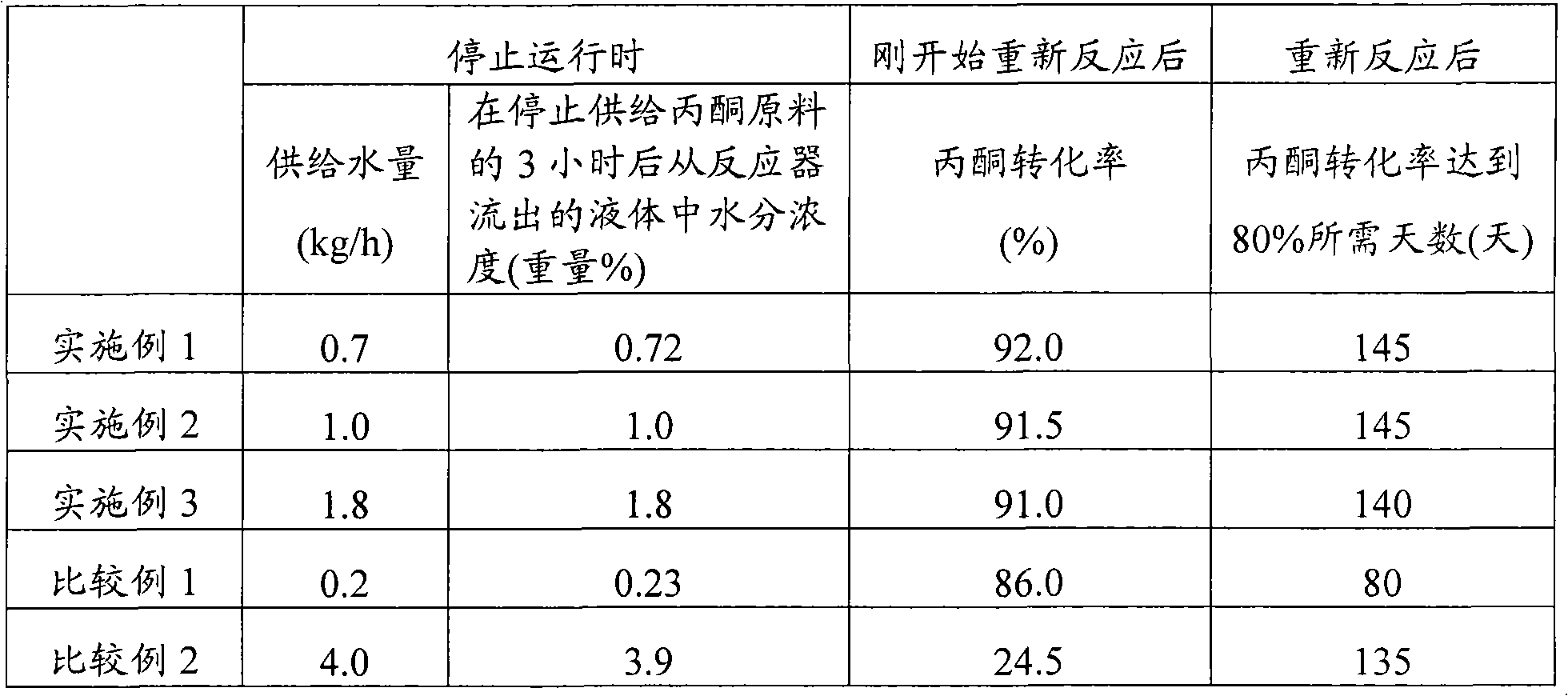
[0033] A fixed bed reactor (bottom radius = 0.2m) was filled with 125L of cation exchange resin. The cation exchange resin uses 2-(4-pyridyl)ethanethiol to convert sulfonic acid type cation exchange resin (manufactured by Mitsubishi Chemical Corporation "ダィャィォンSK104H) ") 15 mol% of the sulfonic acid group is modified to form. A phenol raw material consisting of 86.3% by weight of phenol, 13.7% by weight of other compounds (containing bisphenol A and its isomers, etc.), and 300 ppm by weight of moisture is supplied to the reactor (supply amount = 95 kg / h) , And an acetone raw material composed of 99.7% by weight of acetone and 0.3% by weight of water (supply amount = 3.5 kg / h), reacted at a reaction temperature of 58°C for 180 days. The acetone conversion rate immediately after the reaction started was 98.5%, and the acetone conversion rate after 180 days of operation was 91.5%. At this time, the supply of the acetone raw materi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com