Preparation for external use having improved temporal stability of steroid
A technology of external preparations and steroids, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, non-central analgesics, etc., can solve problems such as poor feeling of use, separation, poor ductility of preparations, etc. To achieve the effect of improving the stability over time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
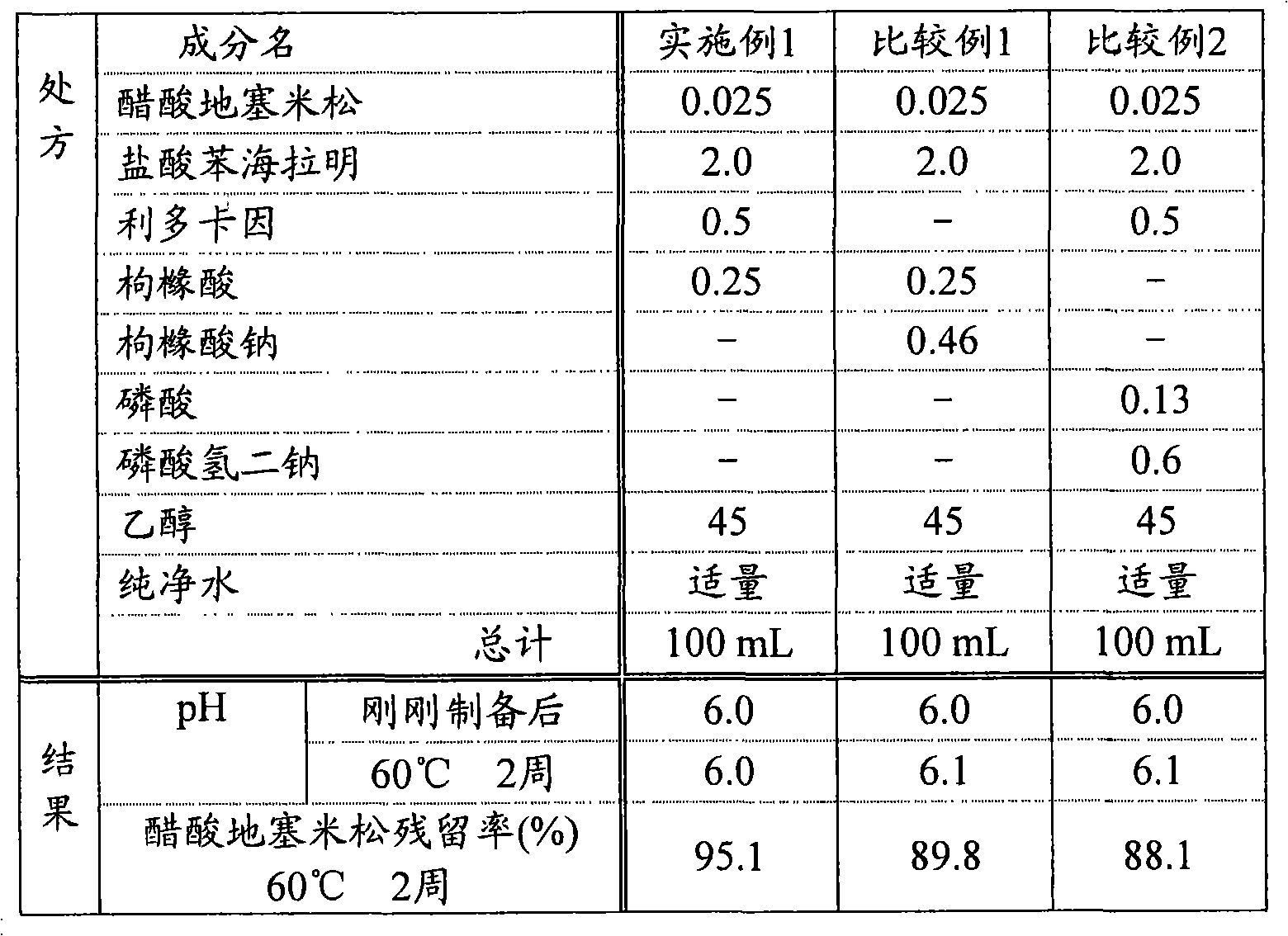
[0051] 0.025 g of dexamethasone acetate and 0.5 g of lidocaine were added to 45 g of ethanol to dissolve to prepare an ethanol solution. Next, 2 g of diphenhydramine hydrochloride and 0.25 g of citric acid were added to 30 g of purified water and stirred to prepare an aqueous solution. After that, the above-mentioned aqueous solution and ethanol solution were mixed, and purified water was added to make the total amount 100 mL to obtain the liquid preparation of Example 1 (pH 6.0).
Embodiment 2
[0062] 0.025 g of dexamethasone acetate and 0.5 g of lidocaine were added to 45 g of ethanol to dissolve to prepare an ethanol solution. Next, 2 g of diphenhydramine hydrochloride and 0.32 g of malic acid were added to 30 g of purified water and stirred to prepare an aqueous solution. After that, the aqueous solution and the ethanol solution were mixed, and purified water was added to make the total amount 100 mL to obtain the liquid preparation of Example 2 (pH 6.06). The pH was 6.09 after storage at 60°C for 10 days. The residual rate of dexamethasone acetate (content after storage at 60°C for 10 days / content immediately after preparation×100) was 96.6%, and the stability of dexamethasone acetate was significantly improved. It should be noted that in the determination of the content of dexamethasone acetate, HPLC was used in the same manner as in Test Example 1.
preparation example 1
[0063] [Preparation Example 1] (Liquid preparation)
[0064] Dexamethasone acetate 0.025% w / v
[0065] Diphenhydramine hydrochloride 2.0%w / v
[0066] Lidocaine 0.5%w / v
[0067] l-menthol 3.0%w / v
[0068] dl-camphor 2.0%w / v
[0069] Citric acid 0.3%w / v
[0070] Ethanol 30%w / v
[0071] Pure water
[0072]
[0073] Total 100mL
[0074] Dexamethasone acetate, l-menthol, dl-camphor and lidocaine are added to ethanol to dissolve to make an ethanol solution. In addition, diphenhydramine hydrochloride and citric acid are added to purified water and stirred to prepare an aqueous solution. After that, the above-mentioned aqueous solution and ethanol solution are mixed to obtain a liquid preparation for external use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com