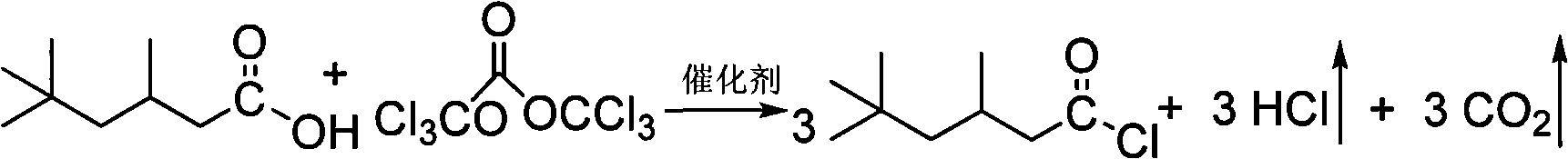
Simple synthetic method of isononanoyl chloride
A technology of isononanoyl chloride and a synthesis method, which is applied in the synthesis of acid chloride compounds and the field of synthesis of isononanoyl chloride, can solve the problems of high requirements on reaction conditions, difficult control of environmental pollution, and high corrosiveness of equipment, and achieves high product purity. , easy to store, transport and use, the effect of less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: in the 50ml four-neck flask that is equipped with thermometer, mechanical stirring, reflux condenser, drying tube and tail gas absorption bottle, at room temperature, under normal pressure, add 4.45g two (trichloromethyl) carbonate solids, Then add 2.37g isononanoic acid and 0.005g morpholine; start stirring, and slowly raise the temperature to 75°C, keep warm, and react until no gas is generated (generally takes about 1 hour); after the reaction solution is cooled, distill under reduced pressure to obtain iso nonanoyl chloride. According to gas chromatography analysis, the yield of isononanoyl chloride is 87.9%, the purity is 98.62%, the appearance is light yellow liquid, b.p.90~92°C / 20kpa.
Embodiment 2
[0015] Embodiment 2: keep the feed ratio of two (trichloromethyl) carbonate solids, isononanoic acid and catalyst morpholine constant, change the solvent added to be chloroform, other operations are all the same as in Example 2, the isononanoyl chloride obtained , analyzed by gas chromatography, the yield of isononanoyl chloride is 77.65%, the purity is 99.32%, the appearance is colorless liquid, b.p.90~92°C / 20kpa.
Embodiment 3
[0016] Embodiment 3: keep the feed ratio of two (trichloromethyl) carbonate solids, isononanoic acid and catalyst morpholine constant, change the solvent that adds is carbon tetrachloride, other operation is all the same as embodiment 2, obtains Isononanoyl chloride, analyzed by gas chromatography, the yield of isononanoyl chloride is 71.1%, the purity is 98.18%, the appearance is colorless liquid, b.p.90~92℃ / 20kpa.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com