Preparation method and application of immunoglobulin antibody with high sialic acid content
An immunoglobulin and sialic acid technology, applied in the direction of antibodies, anti-inflammatory agents, bone diseases, etc., can solve problems such as side effects, and achieve the effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] The following embodiments will further illustrate the present invention in conjunction with the accompanying drawings.
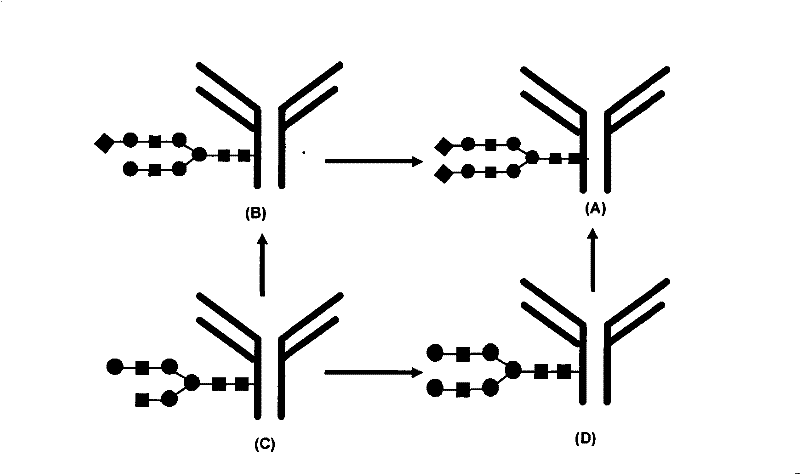
[0015] In 100ml of buffer solution (100mmol phosphoric acid solution, pH 7.5, containing 2mmol divalent manganese ions and 5mmol divalent magnesium ions), add intravenous immunoglobulin G (0.5g), uridine diphosphate-glucose (0.2g), Cytidine-5'-triphosphate (0.2g), sialic acid (0.1g), uridine diphosphate-galactose-4-epimerase enzyme (20 units activity), β1,4-galactosyltransferase ((20 units of activity), cytidine-5'-phosphate-sialic acid synthase (20 units of activity) and α2,6 sialyltransferase (20 units of activity). The reaction solution was stirred slowly at 4°C for 12h. The reaction solution Remove all glycosyltransferases through the His-Tag protein purification column. Then the reaction solution is dialyzed to remove unreacted small molecules such as uridine diphosphate-glucose, buffer solution and metal ions to obtain intravenous immunoglobulin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com