Alkanna tinctoria drug loading nano fiber, preparation and application thereof
A drug-loaded nanometer and nanofiber technology, which is applied in fiber treatment, fiber chemical characteristics, anti-tumor drugs, etc., to achieve the effect of simple preparation process, good biocompatibility, and reduced toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
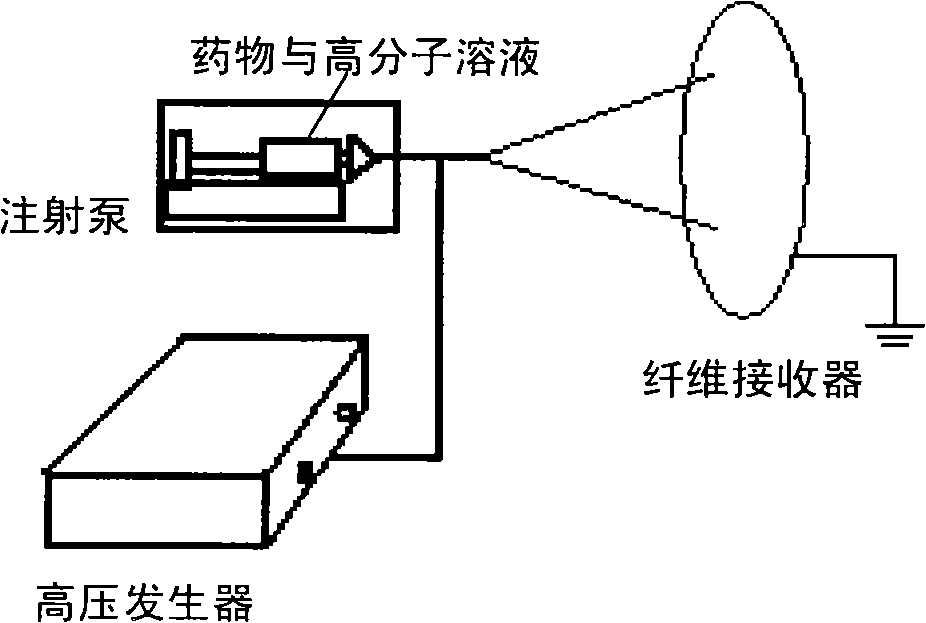
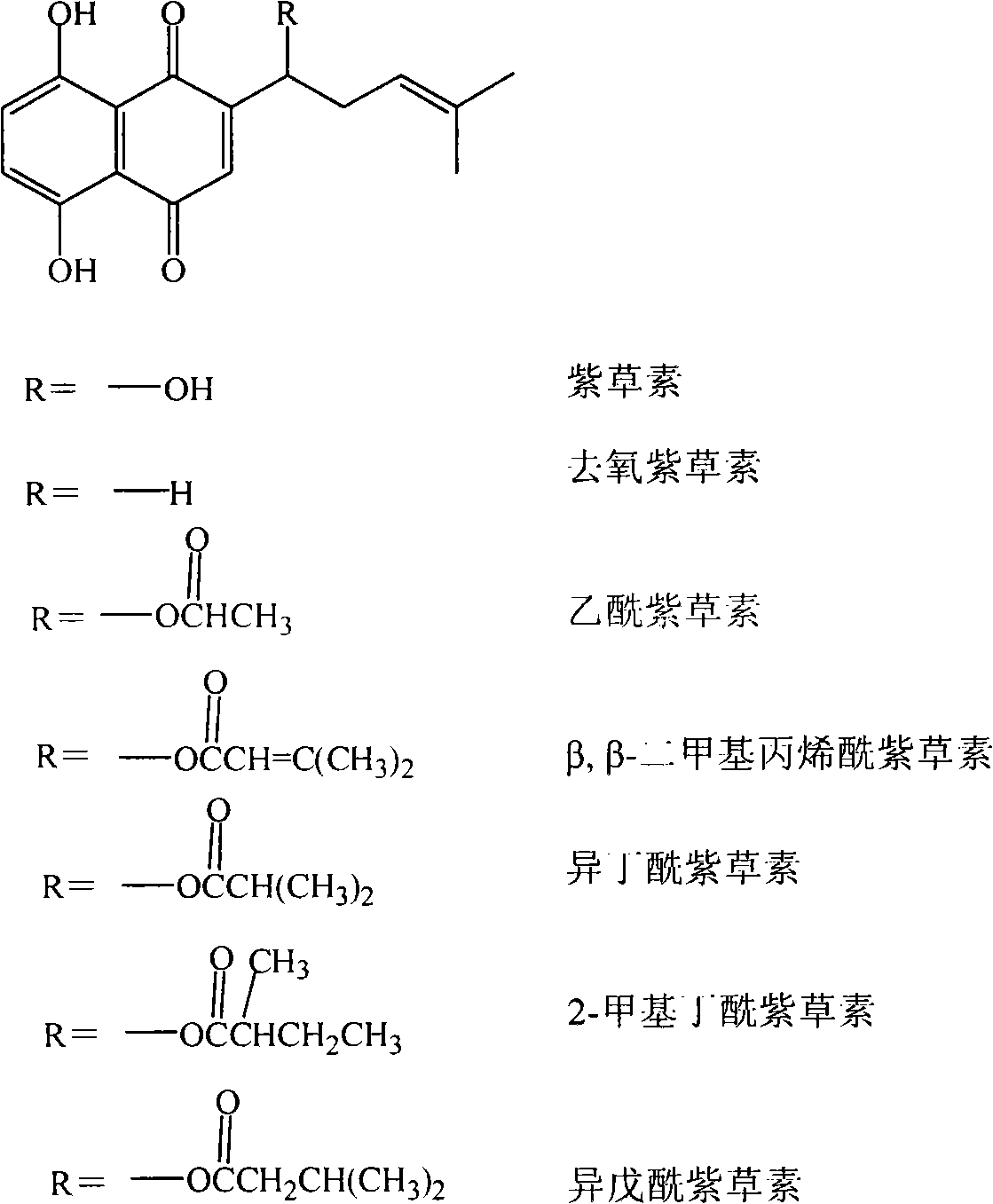
[0026] Dissolve 0.0043g of shikonin in 5mL of chloroform, add 0.4045g of polyεcaprolactone (PCL) (Mw≈100,000) under stirring condition, and ultrasonicate to make it into a homogeneous solution. Pour the prepared solution into a 5mL syringe, and use a right-angle flat-mouth spinneret made of a 9-gauge needle. The flow rate of the solution at the spinneret is 0.6mL / h, the applied voltage is 20kV, and the distance between the two poles is 20cm.
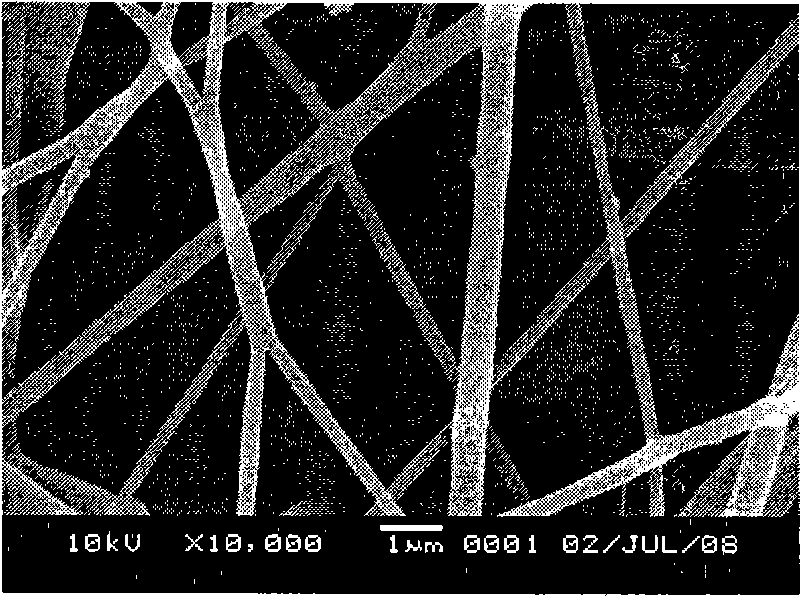
[0027] The result is as figure 1 As shown, the obtained shikonin / PCL drug-loaded fibers have a diameter of about 10 μm. The fiber surface is flat and smooth, and the drug is highly dispersed in the fiber substrate PCL.
Embodiment 2
[0029] Dissolve 0.0204g β, β-dimethylacrylshikonin (β, β-dimethylacrylshikonin) in a mixed solution of 4 mL chloroform and acetone (3:1, v / v), and add 0.4080 g PLGA ( Lactide:glycolide=75:25, Mw≈100,000), sonicated to make a homogeneous solution. Pour the prepared solution into a 5mL syringe, and use a right-angle flat-mouth spinneret made of a No. 8 needle. The flow rate of the solution at the spinneret is 0.8mL / h, the applied voltage is 22kV, and the distance between the two poles is 15cm.
[0030] The obtained β, β-dimethylacryloylshikonin / PLGA drug-loaded fiber has a diameter of about 12 μm. The fiber surface is flat and smooth, and the drug is highly dispersed in the fiber substrate PLGA.
Embodiment 3
[0032] Dissolve 0.0412g of acetylshikonin in 13.8mL of chloroform, add 0.4136g of PLLA (Mw≈100,000) under stirring conditions, and sonicate to make a homogeneous solution. Pour the prepared solution into a 5mL syringe, and use a right-angle flat-mouth spinneret made of a 9-gauge needle. The flow rate of the solution at the spinneret was 1.0 mL / h, the applied voltage was 25 kV, and the distance between the two poles was 15 cm.
[0033] The obtained acetylshikonin / PLLA drug-loaded fiber has a diameter of about 3 μm. The fiber surface is flat and smooth, and the drug is highly dispersed in the fiber substrate PLLA.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fiber diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com