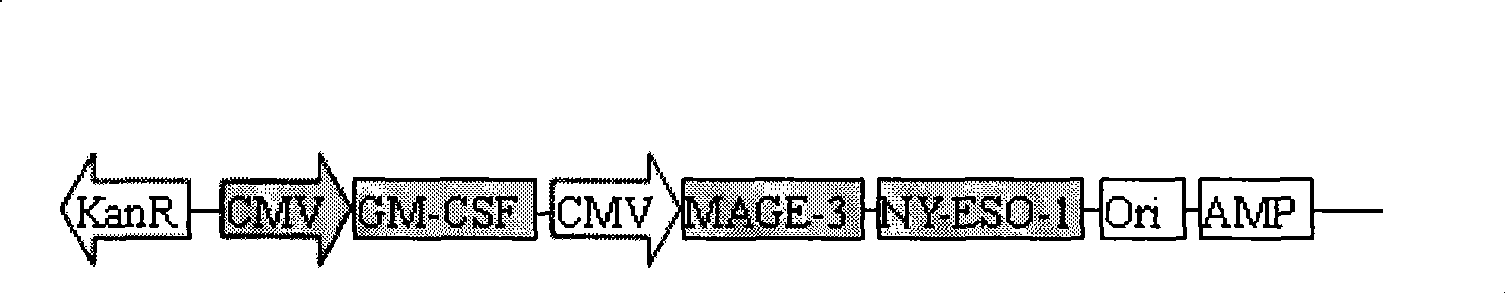Lung cancer DNA plasmid vaccine and preparation method thereof
A plasmid and vaccine technology, applied in the field of DNA vaccines, can solve the problems of insufficient cancer specificity, damage to normal tissues and organs, etc., and achieve the effects of safe and reliable cost, inhibition of development and metastasis, and good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Construction and purification of lung cancer DNA plasmid vaccine: According to the epitopes EVDPIGHLY and FLWGPRALY of lung cancer antigen MAGE-3 recognized by specific CTL and the epitopes SLLMWITQC and QLSLLMWIT of NY-ESO-1, a fusion gene containing the above four epitopes was designed and synthesized , and connect each epitope with flexible tetrapeptide (GPGP) (the size is about 144bp, see the results image 3 ), and subcloned it into the CMV promoter of the pNGVL4a plasmid to obtain the pNGVL-MAGE-NY-ESO plasmid, and inserted the GM-CSF gene fragment of about 432bp obtained by reverse transcription PCR into another part of the above plasmid Under a CMV promoter, constructs such as figure 1 The pNGVL-GM-CSF-MAGE-NY-ESO plasmid shown in the structure; the GM-CSF gene fragment is obtained by conventional methods: first, isolate human peripheral blood leukocytes, extract total RNA, and then design according to the known sequence (Genebank no.AAA52578) Primers, obtained...
Embodiment 2
[0027]Lung cancer DNA plasmid vaccine injection and expression analysis in mammalian cells thereof: the high-purity lung cancer DNA plasmid vaccine pNGVL-GM-CSF-MAGE-NY-ESO obtained in Example 1 and cationic liposome DOSPER (purchased from Roche company ), the mass ratio of DNA plasmid vaccine and cationic liposome is 10:1 and dissolved in phosphate buffer solution to prepare DNA plasmid vaccine injection, and the highly purified plasmid in DNA plasmid vaccine injection is wrapped by cationic liposome .
[0028] Transfect the DNA plasmid vaccine injection into mammalian cos-7 cells, and continue culturing in DMEM medium containing 10% fetal bovine serum at 37 degrees. After harvesting the cultured cells at different periods of time, use flow cytometry (BD company) to analyze the expression of GM-CSF and the cytokines such as IL-4, IL-6 and IFN-γ stimulated by GM-CSF, and confirm that the plasmid stimulates the immune response Ability. The calculation results showed that the ...
Embodiment 3
[0030] Establishment of lung cancer model mice: According to literature reports, BALB / c mouse lung cancer was induced by diethylnitrosamine (DEN): mice were subcutaneously injected with 1% DEN aqueous solution once a week, each dose was 56mg / kg, and the total dose of DEN reached 1176mg , when the observation time is about half a year, the incidence rate of lung cancer is identified by immunohistochemistry, and the general incidence rate can reach 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com