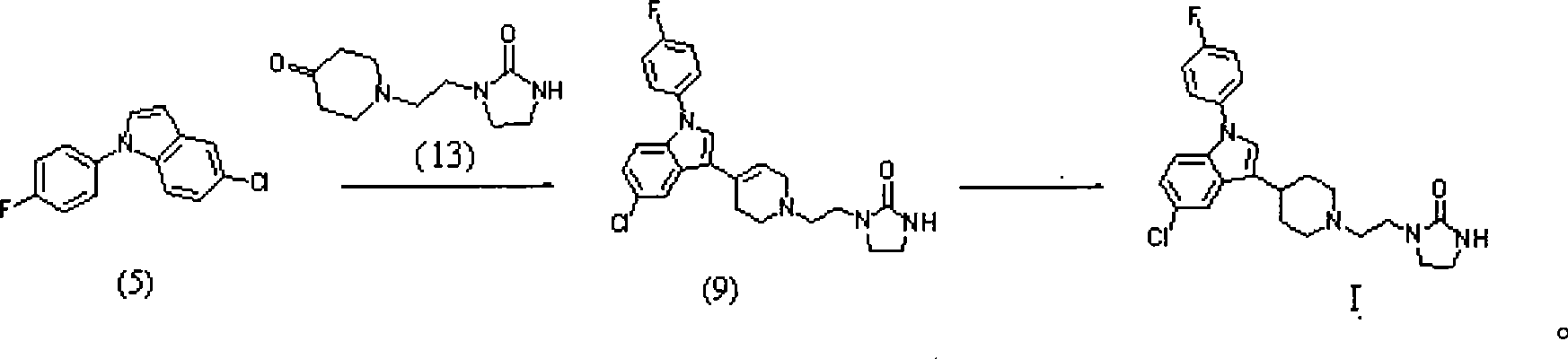
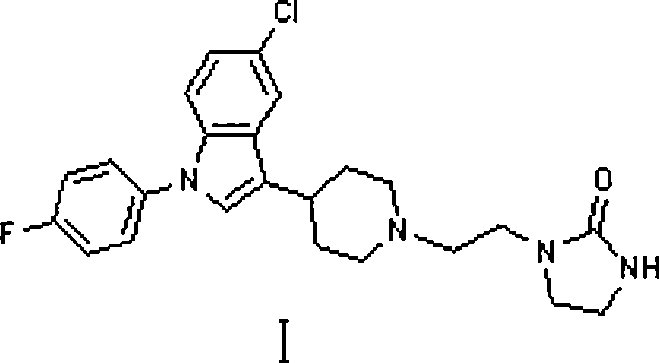
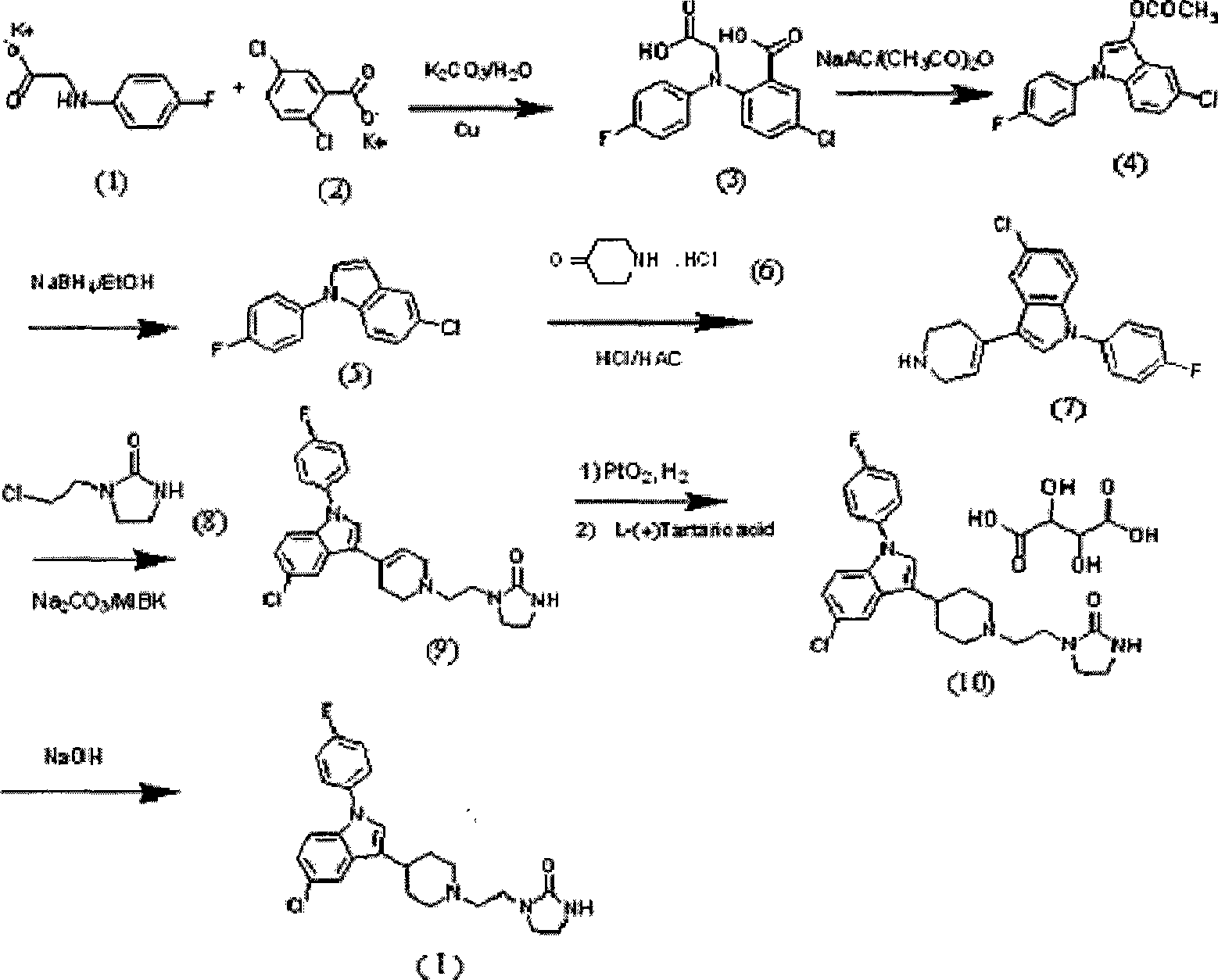
Method for preparing sertindole
A technology of sertindole and compounds, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, cardiovascular system diseases, etc., can solve the problems of high synthesis cost and many reaction steps, and achieve simplified reaction steps and high reaction yield High, beneficial to the effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of (2-chloroethylimidazoline)-2-one (8)
[0037] Put N-(2-hydroxyethyl)ethylenediamine compound (15) (104g, 1mol) and urea (60.06g, 1mol) in a 500ml single-necked bottle, react at 230°C for 4h, and the reaction is complete. Cool, wash 3 times with ether (×150ml), filter, and dissolve the filter cake in 300mlCH 2 Cl 2 In ice bath, slowly add SOCl dropwise 2 (86ml), dropwise, heated to reflux for 3h, quenched the reaction with dilute alkaline water, extracted and separated, the aqueous layer was backwashed with dichloromethane (100ml), the dichloromethane layers were combined, evaporated to dryness, and the resulting solid was diethyl ether ( 80ml) was washed to obtain (2-chloroethylimidazoline)-2-one as light yellow solid 117.80g, and the two-step yield was 79.3%. m.p.86.0~86.4℃. MS (m / z): 149.1 (M+H).
Embodiment 2
[0039] Synthesis of 1-(2-(2-carbonylimidazoline)ethyl)piperidin-4-one (13)
[0040] The raw material (2-chloroethylimidazoline)-2-ketone (19g, 0.128mol), piperidone hydrochloride monohydrate (12) (14g, 0.091mol), NaHCO 3 (23g, 0.273mol) was placed in 120ml of methyl isobutyl ketone, reacted for 24 hours at 95-100°C, filtered, evaporated to dryness, and the product obtained was washed with tetrahydrofuran to obtain 14.09g of the product, with a yield of 63.44%. m.p.115-118°C, MS (m / z): 212.1 (M+H). 1 HNMR (400MHz, CDCl 3 )δ: 2.41(t, 4H), 2.61(t, 2H), 2.78(t, 4H), 3.32(t, 2H), 3.36~3.38(m, 2H), 3.47~3.51(m, 2H), 5.11 (s, 1H).
Embodiment 3
[0042] (2-chloroethylimidazoline)-2-ketone (30g, 0.202mol), piperidone hydrochloride monohydrate (22g, 0.144mol) and NaHCO 3 (36g, 0.432mol) was placed in 200ml MIBK, and reacted at 80°C for 30 hours to obtain 15.3g of compound (13), with a yield of 50.3%. Melting point and spectral data are the same as above.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com