Supports for assaying analytes and methods of making and using thereof
A support and polymer technology, applied in chemical instruments and methods, analytical materials, biological material analysis, etc., can solve problems such as feasibility concerns of anhydride polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
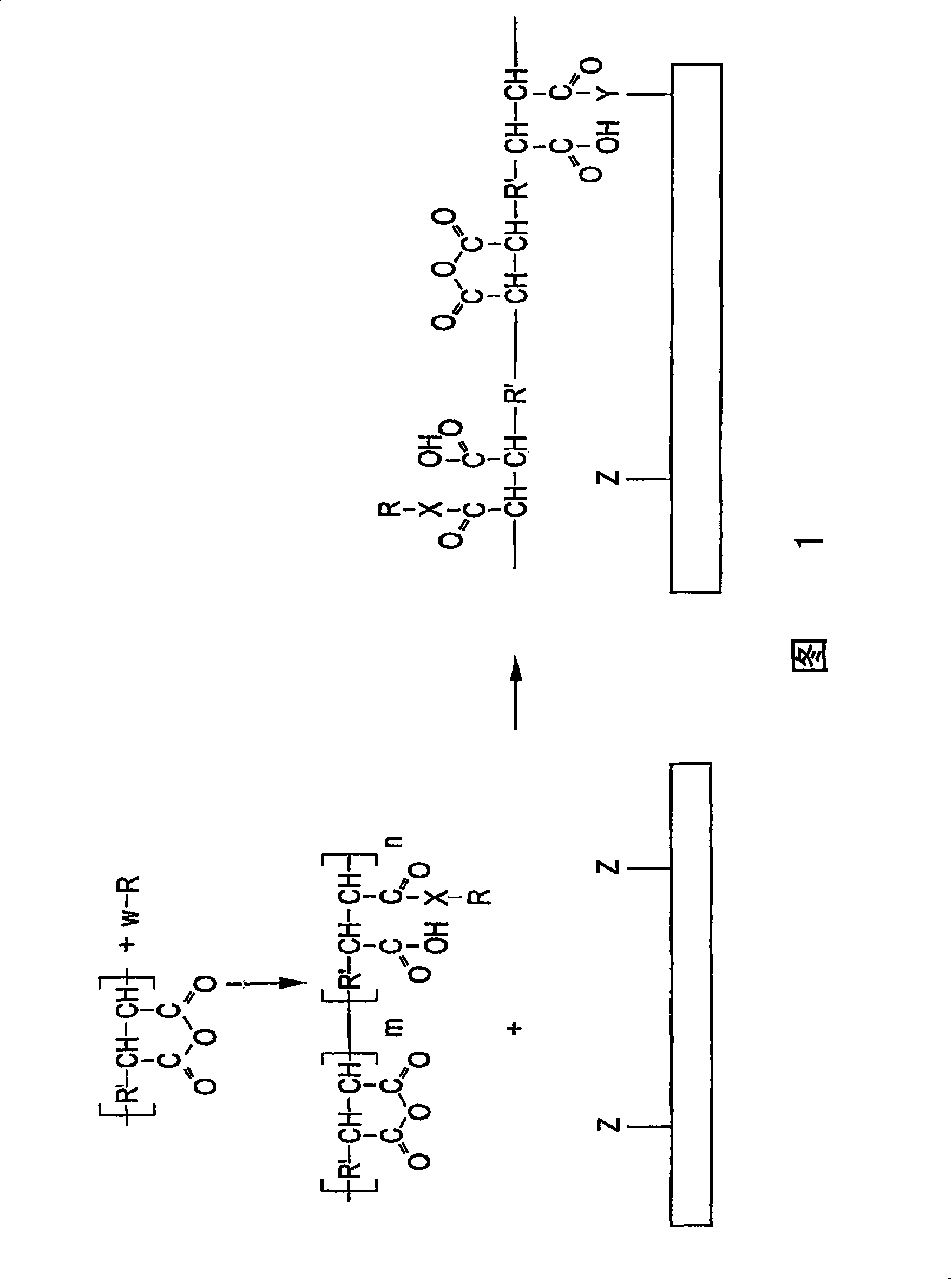
[0065] d. Preparation of support
[0066] The amount of binding polymer attached to the substrate can vary depending on the choice of binding polymer, biomolecule, and analyte to be detected. In one instance, the binding polymer comprises at least a monolayer. In yet another instance, the bonding polymer has a thickness of approximately to about . In another case, the thickness of the bonding polymer has a lower endpoint of 10', 20', 40', 60', 80', 100', 150', 200', 300', 400' or 500', and an upper limit of The endpoints are 750', 1,000', 1,250', 1,500', 1,750', or 2,000', any lower endpoint may form a thickness range with any upper endpoint.
[0067] The binding polymer can be attached to the substrate using techniques well known in the art. For example, the substrate may be dipped in a solution of binding polymer. In another instance, the bonding polymer can be sprayed, vapor deposited, screen printed, or robotically pin printed or embossed onto the substrate. This ...
Embodiment 1
[0104] Example 1. Preparation of ethylene maleic anhydride copolymer pre-terminated with ethanolamine
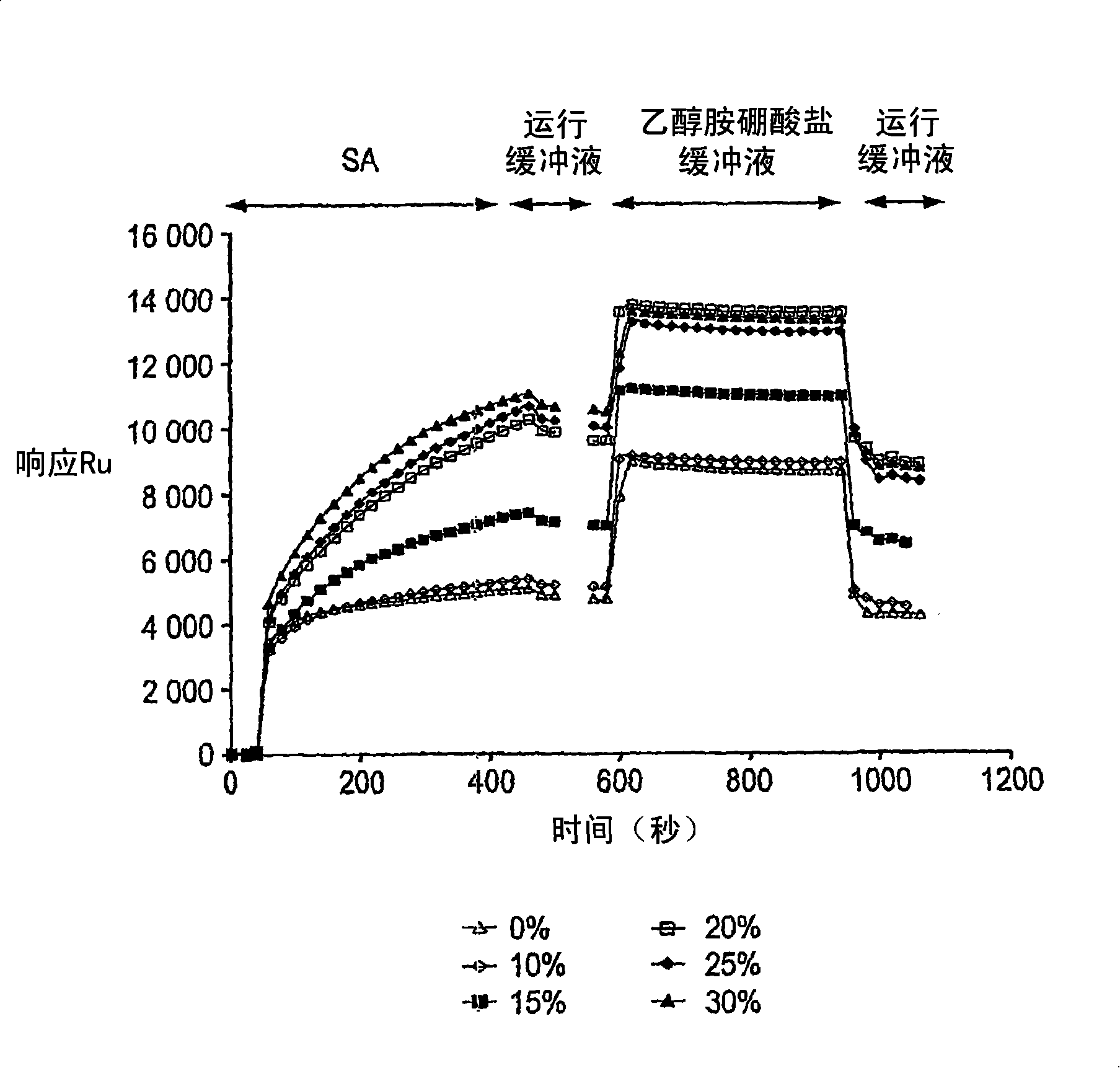
[0105] Figure 13 The FTIR spectra of the (ethylene-maleic anhydride) alternating copolymer (EMA) partially hydrolyzed and dried under vacuum at 140° C. for 1 hour are shown. The EMA spectrum of the partial hydrolysis clearly shows the presence of carboxylic acid groups (located at 1790 cm -1 shoulder), which is due to hydrolysis during storage. The EMA spectrum after vacuum drying showed that all carboxyl groups had been converted to anhydrides (shoulder not visible). This carboxylic acid-free EMA is particularly suitable as a starting material to ensure a well-controlled pre-capping reaction.
[0106] 0.2 g of dry EMA purchased from Sigma Aldrich (ref. 18,805-0) was dissolved in 14.80 g of anhydrous 1-methyl-2- pyrrolidone (NMP). Meanwhile, add 239 μl of pure ethanolamine to 50 g of anhydrous NMP to prepare ethanolamine solution. Then, 5 grams of ethanolamine / NMP sol...
Embodiment 2
[0108] Example 2. Preparation of a gold sensor chip for SPR detection
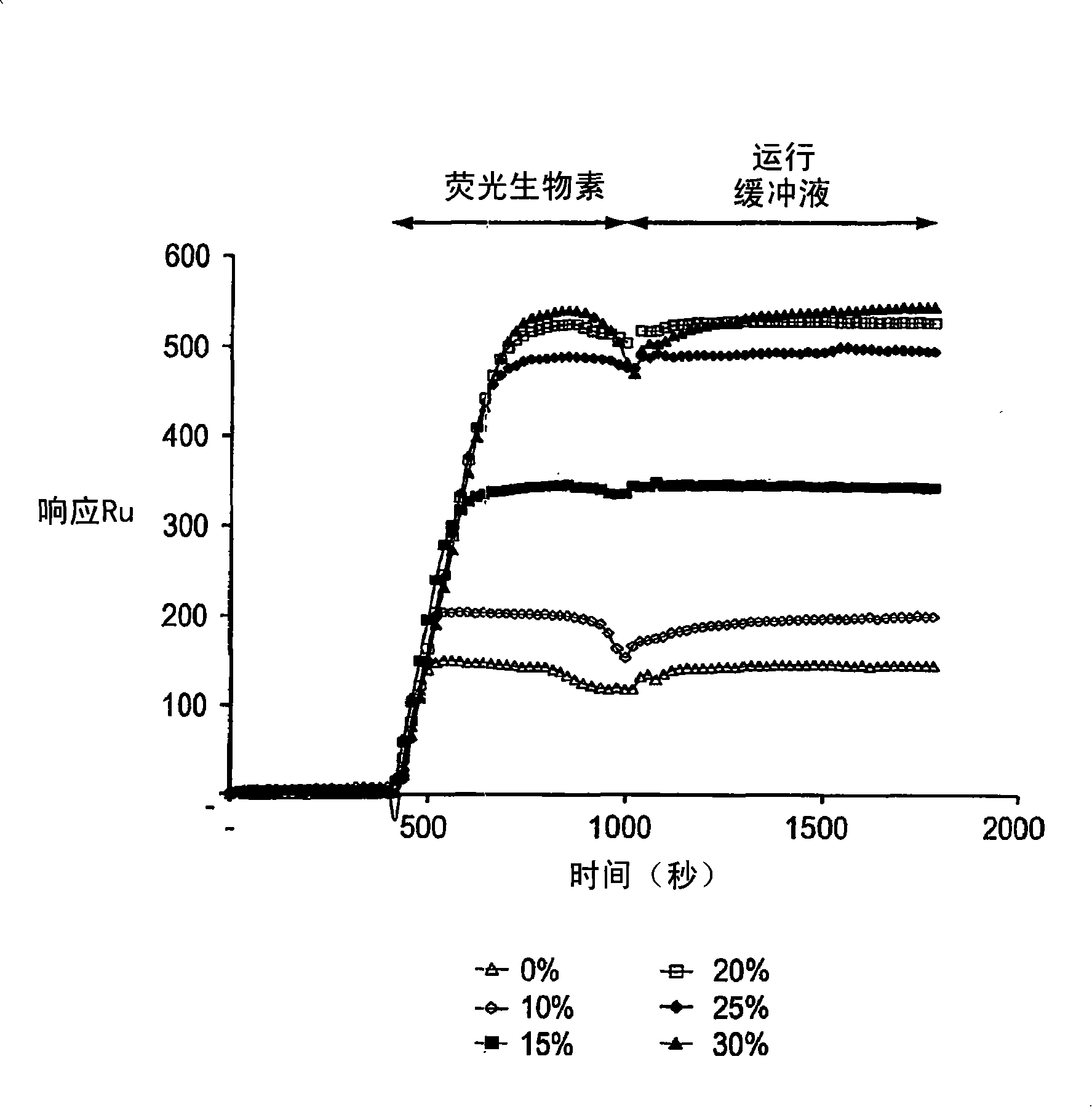
[0109] The Biacore gold sensor chips were cleaned with ethanol and water, then dried in a gentle stream of nitrogen. The chips were soaked in 11-mercaptoundecylamine in 1 mM ethanol solution for 1 hour, washed with ethanol, washed with water, and finally dried in nitrogen flow.
[0110] A silicone rubber gasket (Flexperm) was pasted on each gold chip, and 100 μl of the pre-blocked EMA solution prepared in Example 1 was added to each sample well. After 10 min of coupling, the chips were rinsed with ethanol and dried under nitrogen flow. Remove the silicone gasket after drying. At this point, the coated gold chips are ready to immobilize biomolecules without any activation steps.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com