Tumor-associated antigen 19-9 chemical luminescence immune analytic determination reagent kit and preparation method thereof
A tumor-related antigen, chemiluminescence technology, applied in the field of immunoassay medicine, can solve the problems of radioactive contamination, complicated operation, short storage time of reagents, etc., to achieve specific diagnosis and prognosis judgment, easy operation and production, and ensure the effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
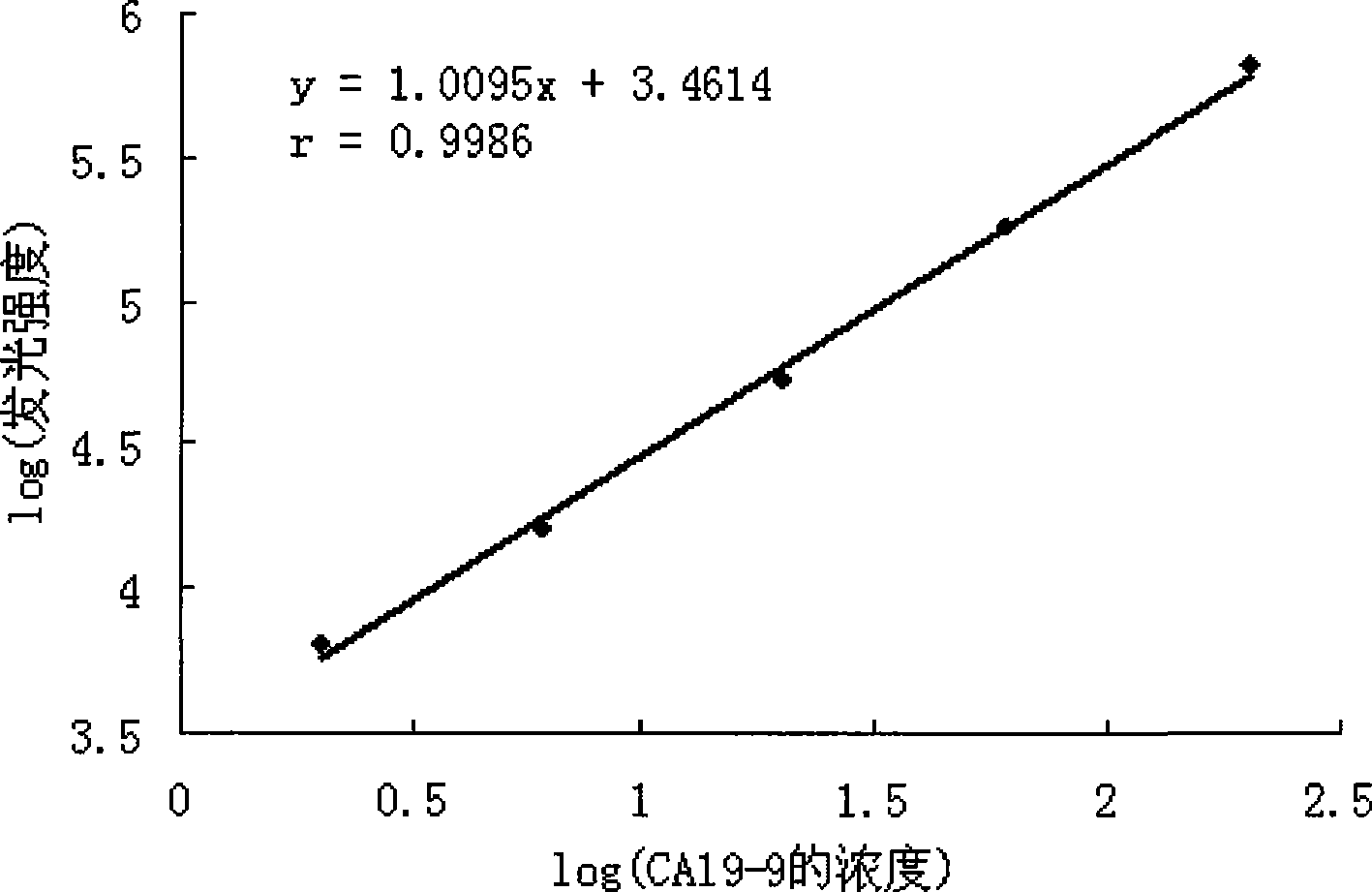
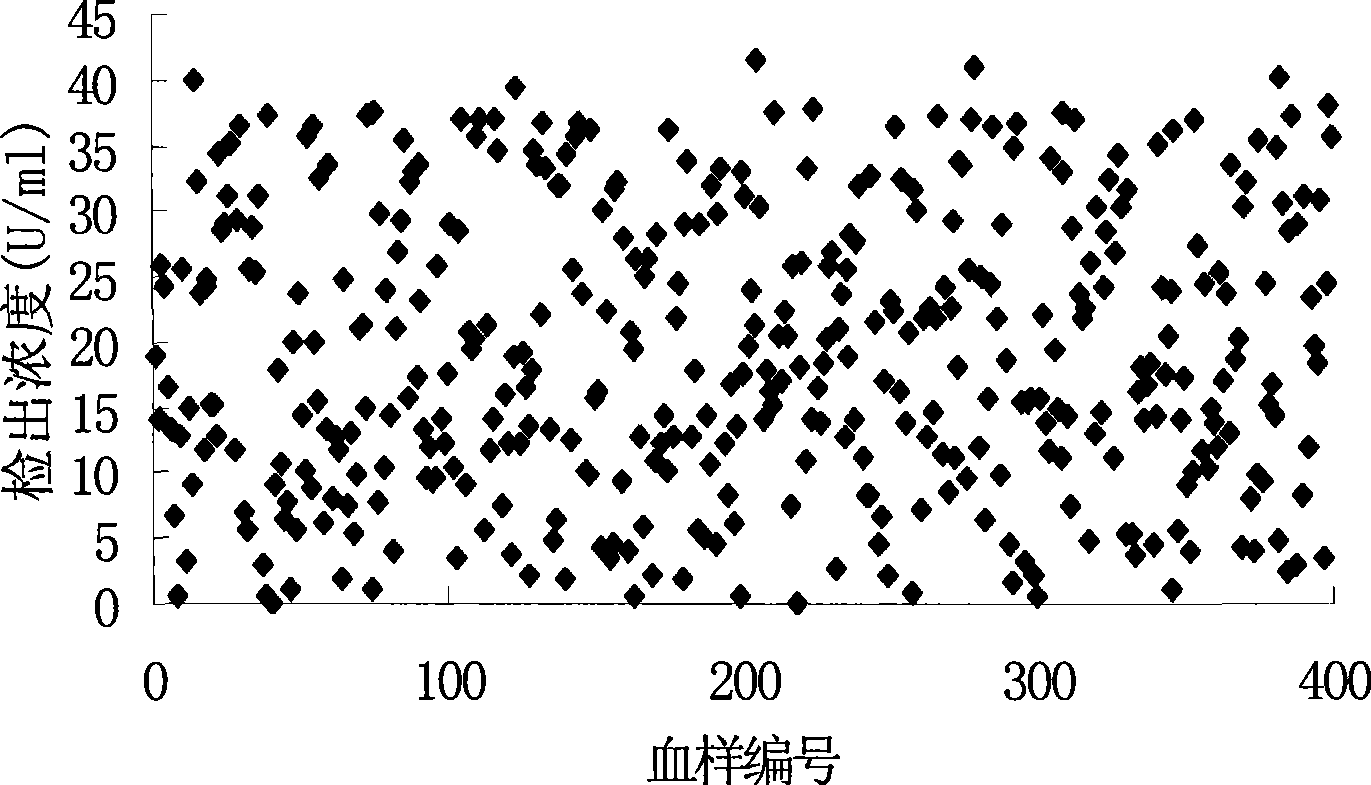
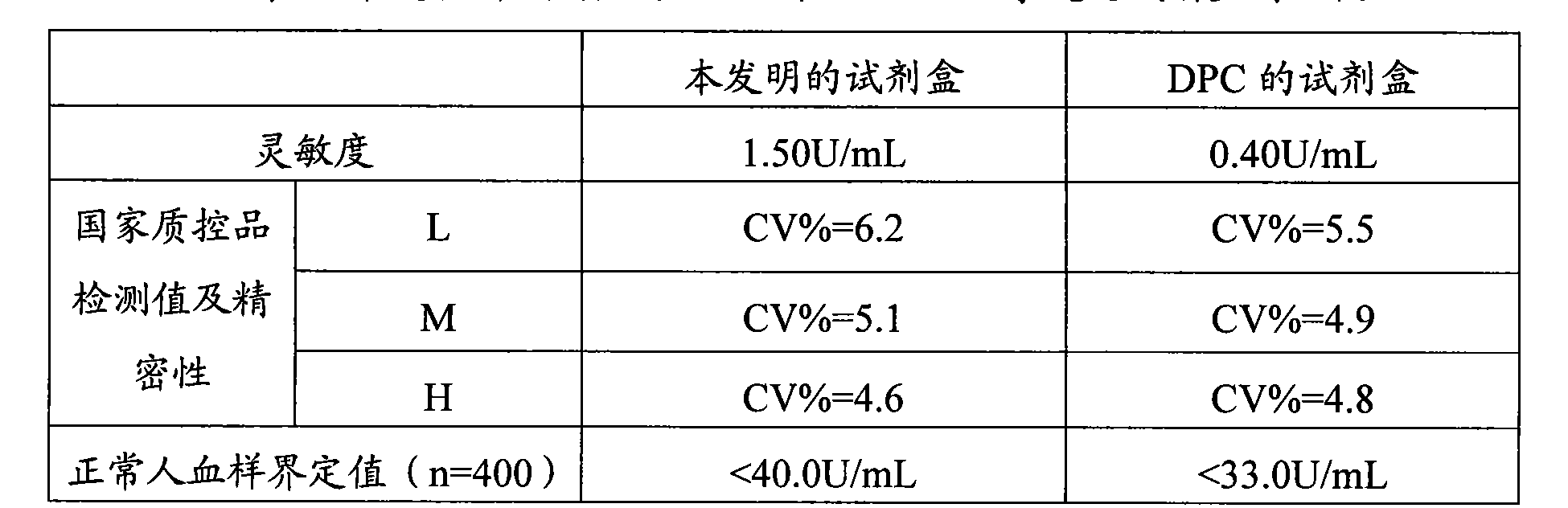
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of the CA19-9 chemiluminescent immunoassay quantitative assay kit of the present invention
[0043] 1. Preparation of enzyme-labeled CA19-9 monoclonal antibody
[0044] The preparation of horseradish peroxidase-labeled CA19-9 monoclonal antibody is as follows:
[0045]Dissolve 4.4mg HRP in 1mL distilled water, add 0.4mL sodium periodate (50mmol / L) and stir at room temperature for 20min, dialyze through 1mmol / L sodium acetate buffer solution, pH4.4, add 8mg CA19-9 monoclonal antibody, stir 2h, finally use 200mmol / L NaBH 4 For reduction, after dialysis with 0.02M PBS buffer, add an equal volume of glycerol, and store below -20°C.
[0046] 2. Preparation of CA19-9 calibrator
[0047] Prepared with pure CA19-9, the concentrations are 0, 2, 6, 20, 60, 200U / mL, 6 bottles in total.
[0048] 3. Enzyme-labeled antibody concentration selection
[0049] The working concentration of enzyme-labeled antibody was selected by square array method as 1:6000.
...
Embodiment 2~4
[0088] Embodiment 2~4 prepares CA19-9 chemiluminescent immunoassay quantitative assay kit of the present invention
[0089] Except that plastic beads, plastic tubes, and magnetic particles were used as carriers, the CA19-9 chemiluminescent immunoassay quantitative assay kit was prepared in the same manner as in Example 1.
Embodiment 5
[0090] Embodiment 5 The using method of kit of the present invention
[0091] The specific operations of the CA19-9 chemiluminescent immunoassay quantitative assay kit prepared in the above example 1 are as follows:
[0092] 1) Take out the kit from the refrigerator at 4°C, and equilibrate at room temperature for 15 minutes;
[0093] 2) Take out the coated slats and insert them into the plate frame;
[0094] 3) Add each concentration of calibrator (0, 2, 6, 20, 60, 200 U / mL) and 25 μL of the sample to be tested in the reaction wells, set a blank well for each experiment, and then add enzyme to each well except the blank well 100 μL of markers, shake and mix thoroughly with a micro shaker, and incubate at 37°C for 1 hour;
[0095] 4) Shake off the reaction solution, fill each well with diluted washing solution, wash the plate 5 times, and finally dry it on clean absorbent paper;
[0096] 5) Add 100 μL of chemiluminescent substrate solution to each well, shake and mix well wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com