Pumpkin protein 2 and preparation method and application thereof
A kind of pumpkin and protein technology, applied in the field of biochemistry, to achieve the effect of eliminating cumbersome steps and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation technology of pumpkin protein 2:
[0028]Take 500g of pumpkin flesh with skin and seeds removed, add 500ml of pH4.5, 10mM acetic acid buffer, homogenize twice on a homogenizer, then adjust pH to 4.0 with 50% glacial acetic acid, and then heat at 16000 at 4°C × g, centrifuged for 30', collected the supernatant, and the supernatant was applied to the 2.6 × 13cm Sp-Sepharose FastFlow column (the column had been balanced with pH4.5, 20mM acetate buffer), and continued to use pH4.5, After washing out a miscellaneous peak with 20mM acetic acid buffer, continue to wash the column with pH6.5, 20mM phosphate buffer, another miscellaneous peak will be washed out, and then use 0-0.6M NaCl (at pH6.5, 20mM phosphate buffer solution) gradient elution to collect the first peak. Mix the first peak collection solution, adjust the pH4 with 50% glacial acetic acid, add 2 times the volume of pH4.5, 20mM acetic acid buffer, add the sample to the 1.6 × 13cm Sp-...
Embodiment 2
[0029] Embodiment 2: Mono-S analysis of strong cation prepacked column:
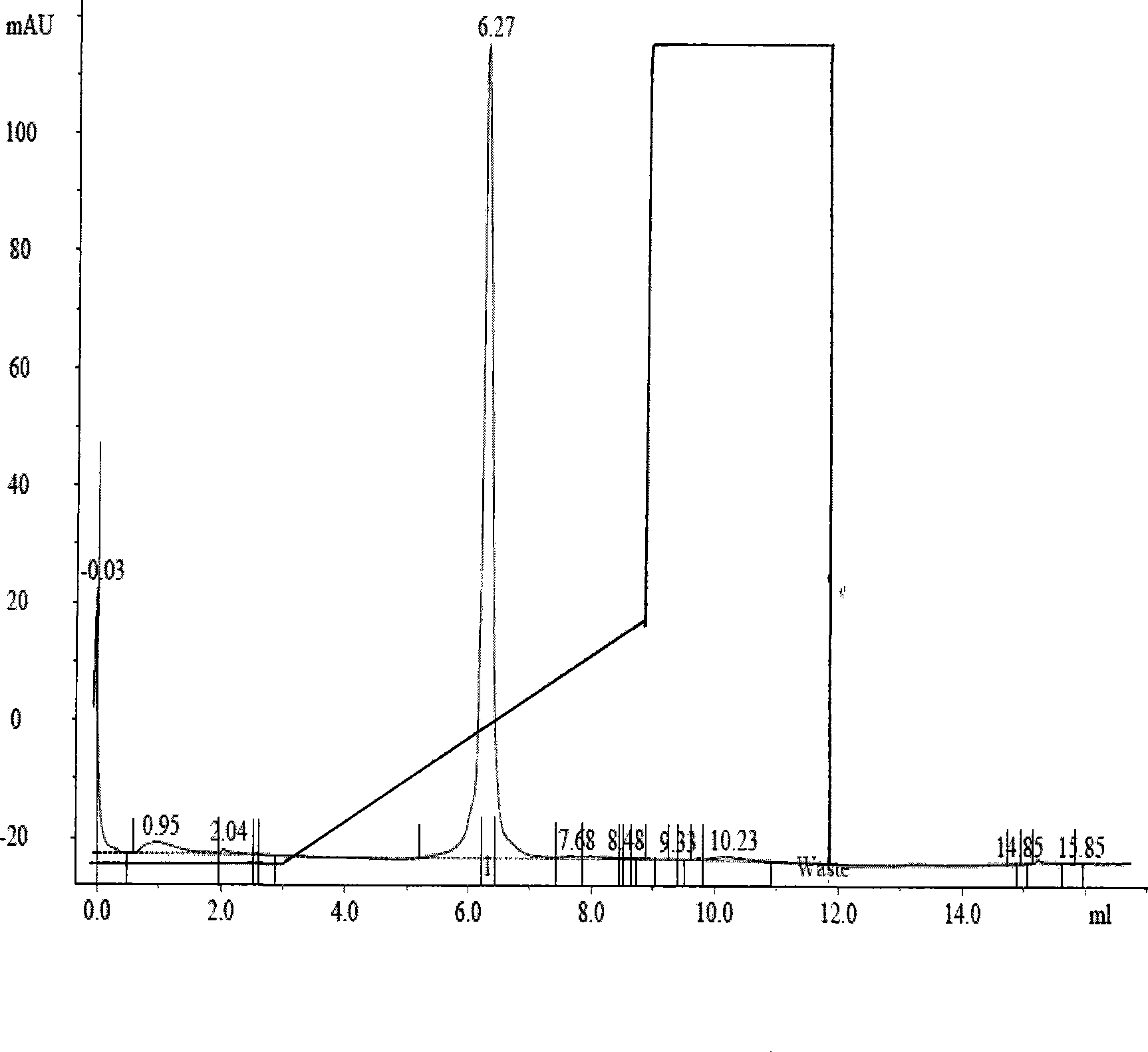
[0030] The elution chart of pumpkin protein 2 isolated and purified according to the present invention on the Mono-S HR5 / 5 column (see attached figure): liquid A is pH7.5, 10mM phosphate buffer, liquid B is liquid A+1M NaCl, and the flow rate is 1ml / min, after the Mono-S column is equilibrated with solution A, add sample, wash the column with 3ml of solution A, elute 6ml with a linear gradient of 0-30% solution B, then wash 3ml with 100% solution B, and then equilibrate 5ml with solution A. Pumpkin protein 2 peaked when the eluent reached 6.27ml, that is, when the NaCl concentration was 0.16M.
Embodiment 3
[0031] Example 3: Crystal Growth of Pumpkin Protein 2:
[0032] The pumpkin protein 2 purified by the present invention is concentrated to 14mg / ml, the pool solution is pH5.4, 0.1M citric acid buffer pH6.0-7.8 or 0.1M phosphate buffer, 28% PEG6K is used as a precipitating agent, and the hanging drop method is used Single crystals grow. The single crystal was collected at a synchrotron radiation source for a set of Diffraction data, the crystal belongs to P2 1 2 1 2 1 crystal form, the unit cell parameters are
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com