Main allergic protein of humulus pollen
A technology for pollen allergy and humulus, which is applied in the field of main allergenic proteins of humulus pollen, can solve the problems of limited research on allergenic proteins of humulus pollen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
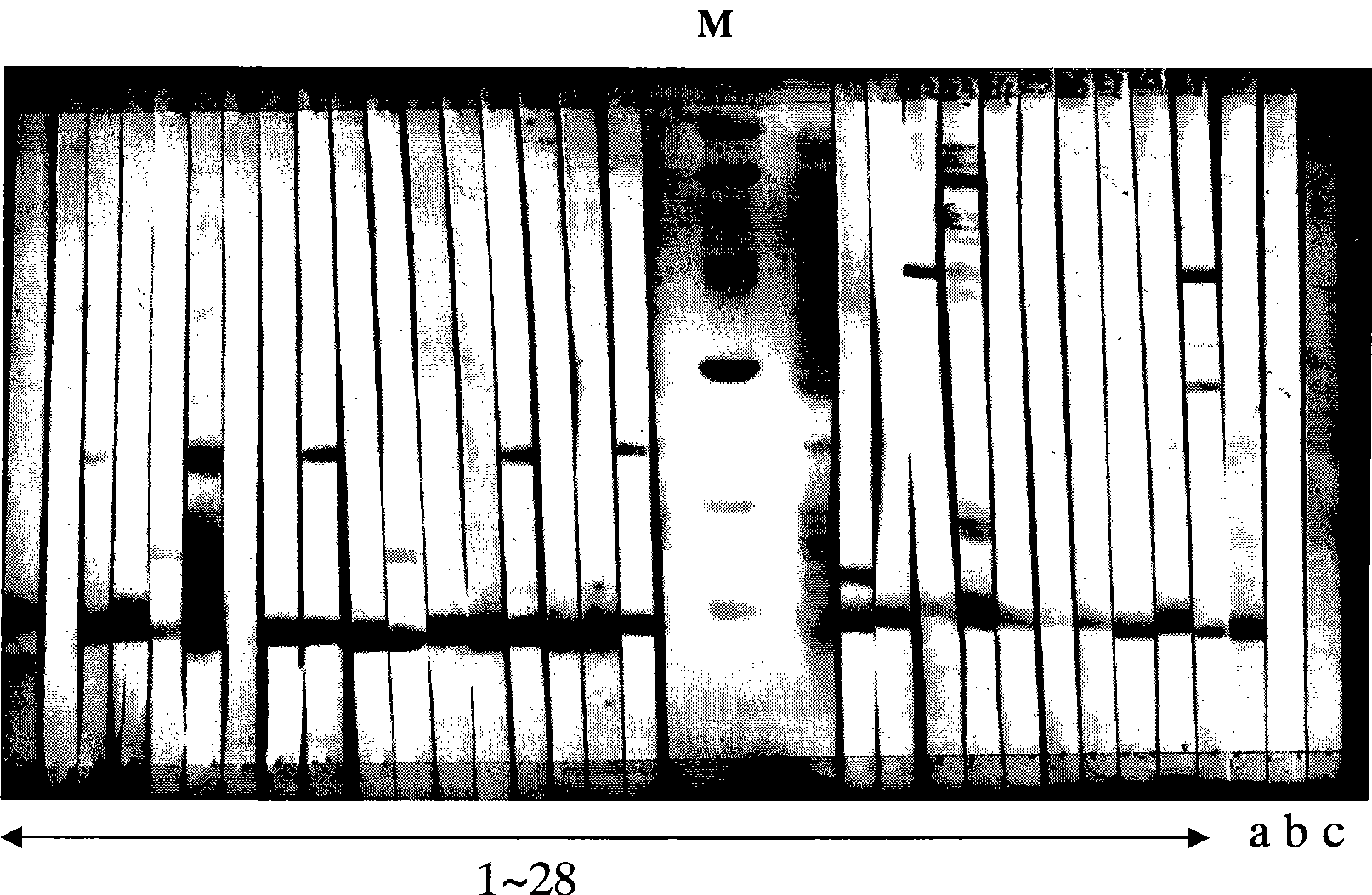
[0030] Example 1 Determination of major allergenic proteins in pollen of Humulus japonicus
[0031]1. Collection of serum from Humulus pollinosis patients and healthy volunteers: There are strict selection criteria for the collection of serum from Humulus pollinosis patients and healthy volunteers. A total of 28 patients with humulus pollinosis were collected in this study. The selection criteria for patients are as follows: 1) Typical history of pollen allergy in summer and autumn: seasonal rhinitis, conjunctivitis and / or bronchial asthma, during summer and autumn (July to October) ) disease; 2) Intradermal test of Humulus pollen extract ≥ ++ At the same time, Pharmacia CAP system RAST FEIA detected Humulus pollen sIgE ≥ grade II (0.70kU / l). At the same time, the serum of 10 healthy volunteers was collected in this study. The inclusion criteria were: no history of allergic diseases (including drug, food allergy, allergic rhinitis, bronchial asthma, and other allergic diseases...
Embodiment 2
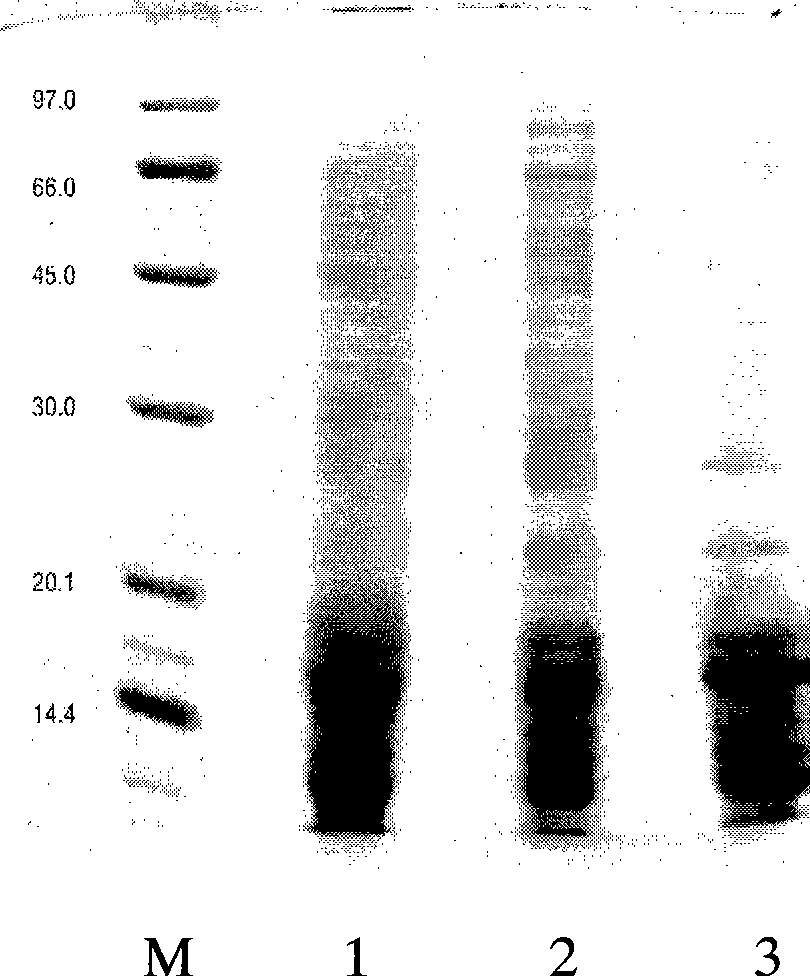
[0034] Example 2 Separation and purification of main allergenic proteins
[0035] 1. Gel size exclusion chromatography
[0036] Chromatography system: Purifier10 (Amersham Company)
[0037] Chromatographic column: Sephacryl 100 prepacked column (Amersham company)
[0038] Column volume 120ml
[0039] Length×Diameter: 60cm×16mm
[0040] Separation range: 10-100kDa
[0041] 1) Sample preparation:
[0042] a. Degrease the freshly collected Humulus pollen with acetone and dry.
[0043] b. Extract (1 / 20w / v) with 0.01M PBS (pH8.0) magnetic force and gentle stirring for 24hr at 4°C.
[0044] c. Centrifuge at 10000 g for 30 min (4° C.) to obtain a supernatant.
[0045] d. Concentrate the supernatant to a protein concentration of at least 1 mg / ml, carry out 80% saturation ammonium sulfate salting out precipitation, centrifuge at 5000g×30min, discard the supernatant and redissolve the precipitated protein in 0.01M PBS (pH8. 0) buffer solution, and use the buffer solution for ...
Embodiment 3
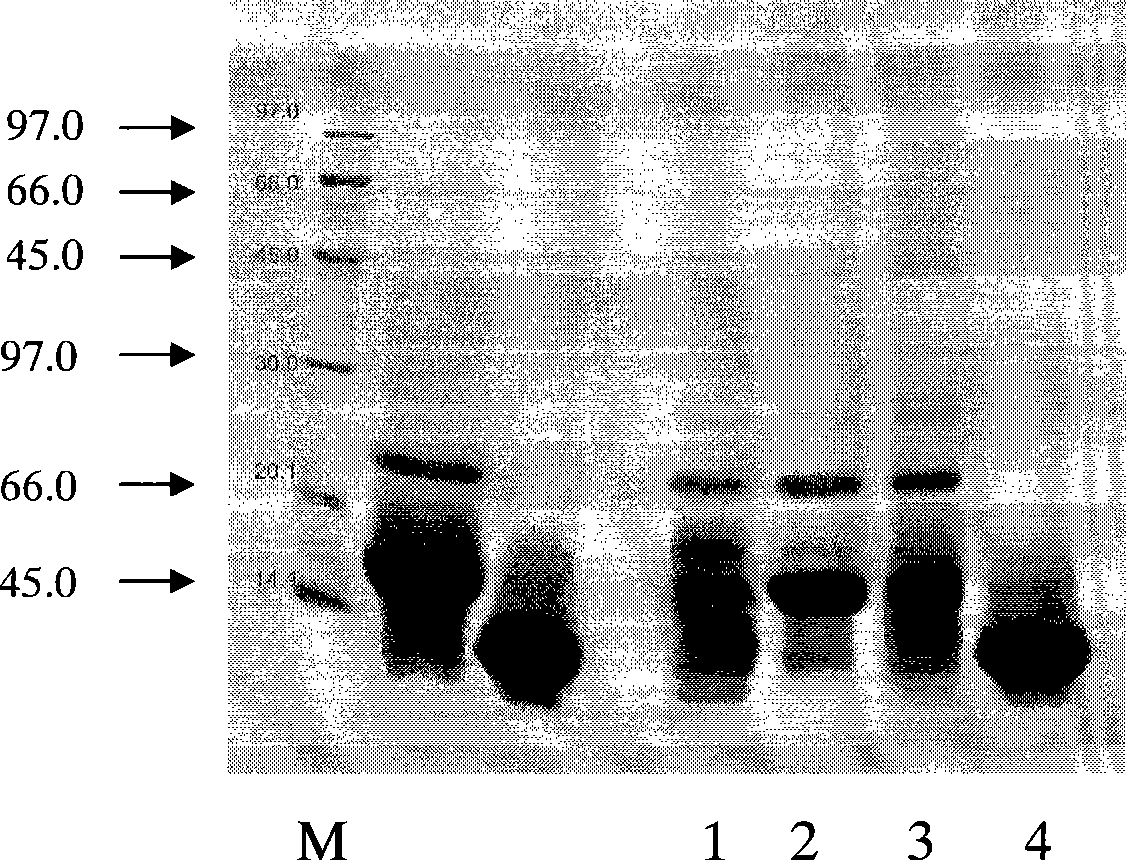
[0062] Example 3 Preparation of Monoclonal Antibody to Major Allergenic Protein of Humulus pollen
[0063] For specific operations, refer to Chapter 11: Hybridoma Technology and Monoclonal Antibody Preparation in "Contemporary Immunology Technology and Application" edited by Bardenian. The operation process is briefly described as follows:
[0064] 1) Immunization of animals Three 6-week-old female healthy BALB / c mice were immunized at the same time. For the first immunization, each mouse was injected with 10-15 μg of antigen. Mix Freund's complete adjuvant with 100 μl of antigen in an equal volume, mix thoroughly with a micro-stirrer, and inject intraperitoneally.
[0065] 2) The second immunization is carried out 2 to 4 weeks later, also known as booster immunization. The amount of antigen for booster immunization was halved, and Freund's incomplete adjuvant was used instead, but the injection volume and method remained unchanged. A total of 4 booster immunizations were ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com