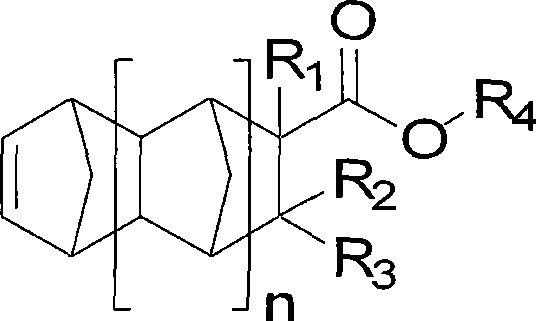
Norbornene-ester polymer containing bulky substituents
A norbornene and polymer technology, applied in the field of norbornene-ester polymers, can solve problems such as difficult to ensure and unsuitable, and achieve the effects of improving light transmittance, good metal adhesion, and excellent optical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0053] The present invention can be better understood through the following examples, which are set forth for illustration and should not be construed as limiting the present invention.
[0054] Synthesis of Norbornene-Ester Monomer (Synthesis Examples 1-8, Comparative Synthesis Examples 1-2)
Synthetic example 1
[0055] Synthesis of 2-methyl-2-adamantyl-5-norbornene-2-methyl-2-carboxylate
[0056] Into a 0.25 L autoclave, DCPD (dicyclopentadiene, Aldrich, 10.2 ml, 0.0757 mol), 2-methyl-2-adamantyl methacrylate (42.6 g, 0.18 mol) and hydroquinone ( 0.83g, 0.1mol), then it was reacted at 180°C for 12 hours, after the reaction product was cooled, it was transferred to a distillation apparatus, and then a vacuum pump was used to distill under a reduced pressure of 1 torr, thereby obtaining the final product at 110°C (product rate: 25%). The molar ratio (mol%) of exo isomer to endoisomer of this product was 48.5:51.5.
[0057] 1 H-NMR (500MHz, CDCl 3 ), inner shape: δ 6.20(dd, 1H), 6.18(dd, 1H); outer shape: δ 6.12(m, 2H)
Synthetic example 2
[0058] Synthesis of 2-ethyl-2-adamantyl-5-norbornene-2-methyl-2-carboxylate
[0059] Into a 0.25 L autoclave, DCPD (dicyclopentadiene, Aldrich, 10.2 ml, 0.0757 mol), 2-ethyl-2-adamantyl methacrylate (44.3 g, 0.18 mol) and hydroquinone ( 0.83g, 0.1mol), then it was reacted at 200°C for 12 hours, after the reaction product was cooled, it was transferred to a distillation apparatus, and then a vacuum pump was used to distill under a reduced pressure of 1 torr, thereby obtaining the final product (product) at 120°C rate: 27%). The molar ratio (mol%) of exo isomer to endo isomer of this product is 45.5:54.2.
[0060] 1 H-NMR (500MHz, CDCl 3 ), inner shape: δ 6.22(dd, 1H), 6.19(dd, 1H); outer shape: δ 6.18(m, 2H)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com