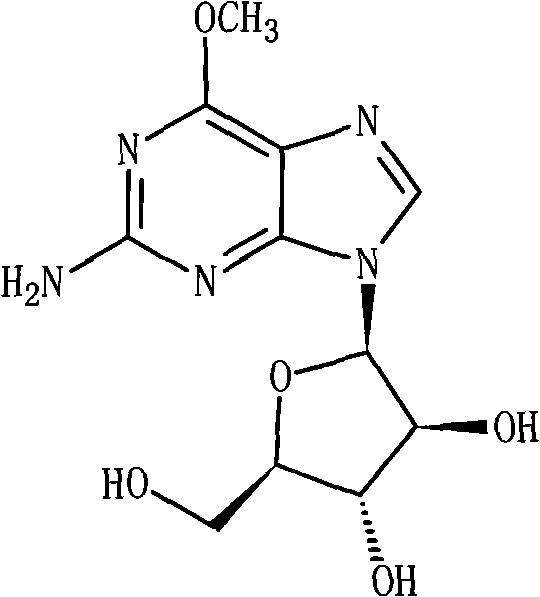
Nelarabine injection
A technology for injection and water for injection, which is used in drug delivery, organic active ingredients, medical preparations containing active ingredients, etc. High temperature sterilization, toxic and side effects degradation products, etc., to achieve considerable economic and social benefits, high sterility assurance level, and low content of related substances.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of Nelarabine Injection by Ordinary Method (1)
[0023] prescription
[0024] Nalarabine 250g
[0026] Add water for injection to 50000g
[0027]
[0028] A total of 1000 bottles were made
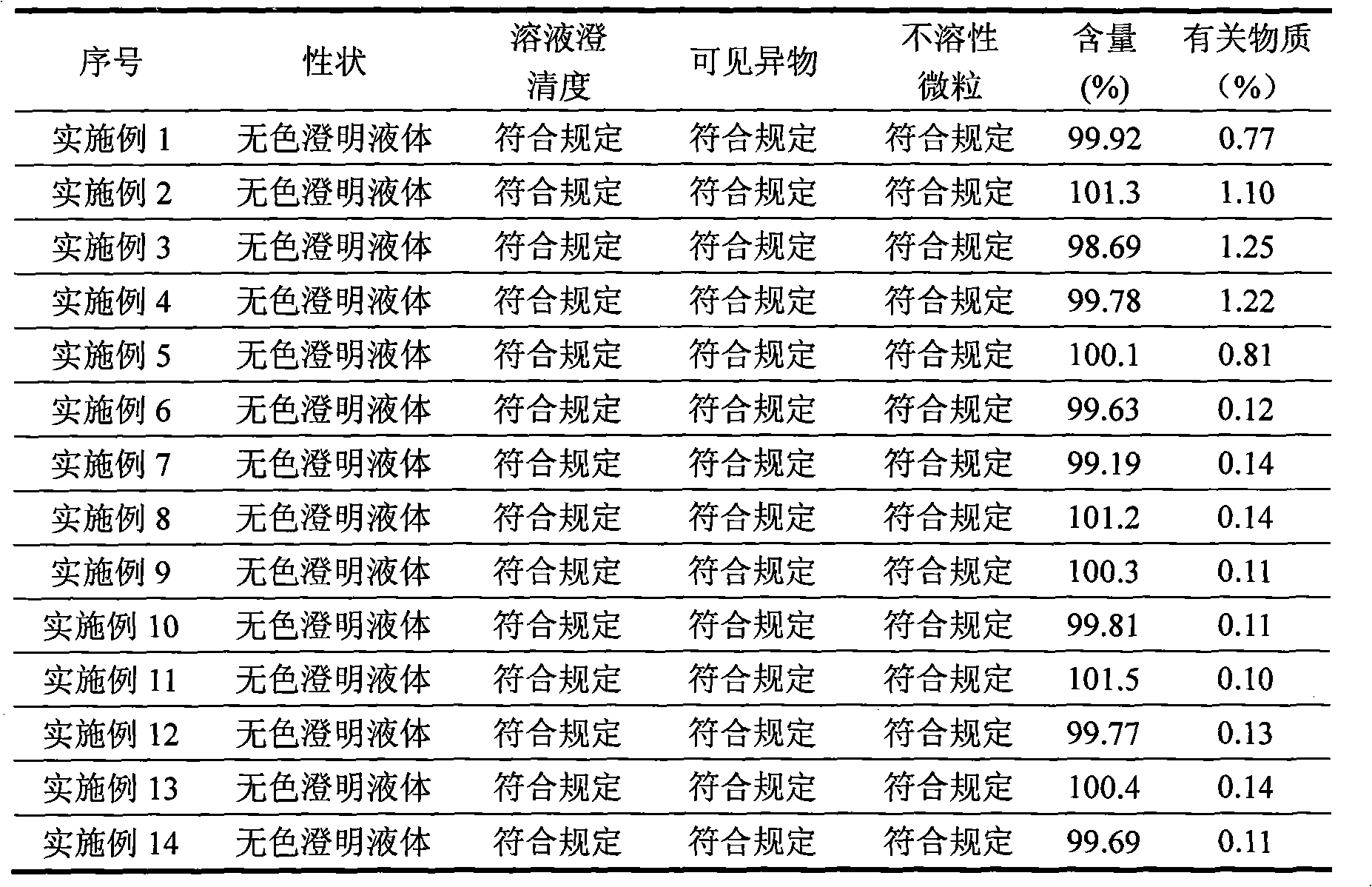
[0029] Take 80% of the water for injection and put it in a stainless steel bucket, weigh the raw and auxiliary materials according to the prescription and add them to the water for injection, stir for ten minutes to fully dissolve the raw and auxiliary materials and mix evenly, add 1mol / L hydrochloric acid solution or 1mol / L sodium hydroxide solution Adjust the pH value to 6.1, add 0.02% activated carbon for injection, keep the water temperature at 70°C, stir for 30 minutes, filter and decarbonize while it is hot, let cool to room temperature, measure the solution content and pH value, add water for injection to the full amount according to the measurement results, and mix Evenly, filter through a 0.22 μm micr...
Embodiment 2
[0031] Preparation of Nelarabine Injection by Common Method (2)
[0032] prescription
[0033] Nalarabine 250g
[0034] Sodium chloride 225g
[0035] Add water for injection to 50000g
[0036]
[0037] A total of 1000 bottles were made
[0038] Take 80% of the water for injection and put it in a stainless steel bucket, weigh the raw and auxiliary materials according to the prescription and add them to the water for injection, stir for ten minutes to fully dissolve the raw and auxiliary materials and mix evenly, add 1mol / L hydrochloric acid solution or 1mol / L sodium hydroxide solution Adjust the pH value to 6.0, add 0.02% activated carbon for injection, keep the water temperature at 70°C, stir for 30 minutes, filter and decarbonize while it is hot, let cool to room temperature, measure the solution content and pH value, add water for injection to the full amount according to the measurement results, and mix Evenly, filter through a 0.22 μm micropor...
Embodiment 3
[0040] Preparation of Nelarabine Injection by Ordinary Method (3)
[0041] prescription
[0042] Nalarabine 250g
[0043] Sodium chloride 225g
[0044] Sodium bisulfite 20g
[0045] Add water for injection to 50000g
[0046]
[0047] A total of 1000 bottles were made
[0048] Take 80% of the water for injection and put it in a stainless steel bucket, weigh the raw and auxiliary materials according to the prescription and add them to the water for injection, stir for ten minutes to fully dissolve the raw and auxiliary materials and mix evenly, add 1mol / L hydrochloric acid solution or 1mol / L sodium hydroxide solution Adjust the pH value to 6.3, add 0.02% activated carbon for injection, keep the water temperature at 70°C, stir for 30 minutes, filter and decarbonize while it is hot, let cool to room temperature, measure the solution content and pH value, add water for injection to the full amount according to the measurement results, and mix Evenl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com