Preparation of semicarbazide derivative hapten, antigen and antibody
A semicarbazide and derivative technology, applied in the field of immunochemistry, can solve the problems that there are no technical reports on semicarbazide derivative hapten, antigen and its specific antibody, and achieve high titer and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 The preparation method of semicarbazide derivative hapten MCPSEM
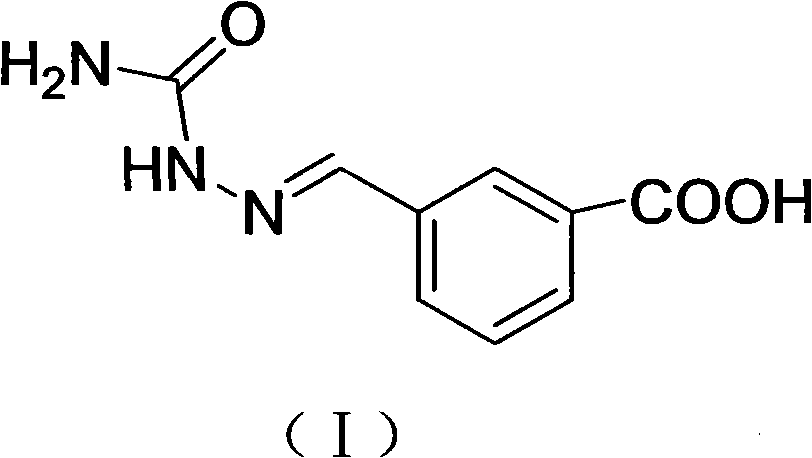
[0037] Add 1.15g of m-carboxybenzaldehyde into a 50ml round bottom flask, slowly add methanol until the p-carboxybenzaldehyde is completely dissolved, add 0.3g of semicarbazide during stirring, and stir overnight at room temperature; after the reaction is completed, filter, wash twice with water, and wash twice with methanol Repeatedly, dry, obtain 0.62g white powder, its structure is as shown in formula (I). The APCI-MS molecular ion peak of hapten MCPSEM is 207, HNMR (600MHz, d 6 -DMSO, TMS): δ10.35(s, 1H); 8.14(s, 1H); 8.03(d, J=7.8Hz, 1H); 7.93(s, 1H); 7.90(d, J=7.8Hz, 1H); 7.52 (t, J = 7.2 Hz, 1H,). The above spectral data can be assigned correctly, which is consistent with the structure of MCPSEM, indicating that the hapten MCPSEM was synthesized successfully.
[0038]
Embodiment 2
[0039] The preparation method of embodiment 2 semicarbazide derivative hapten CEPSEM
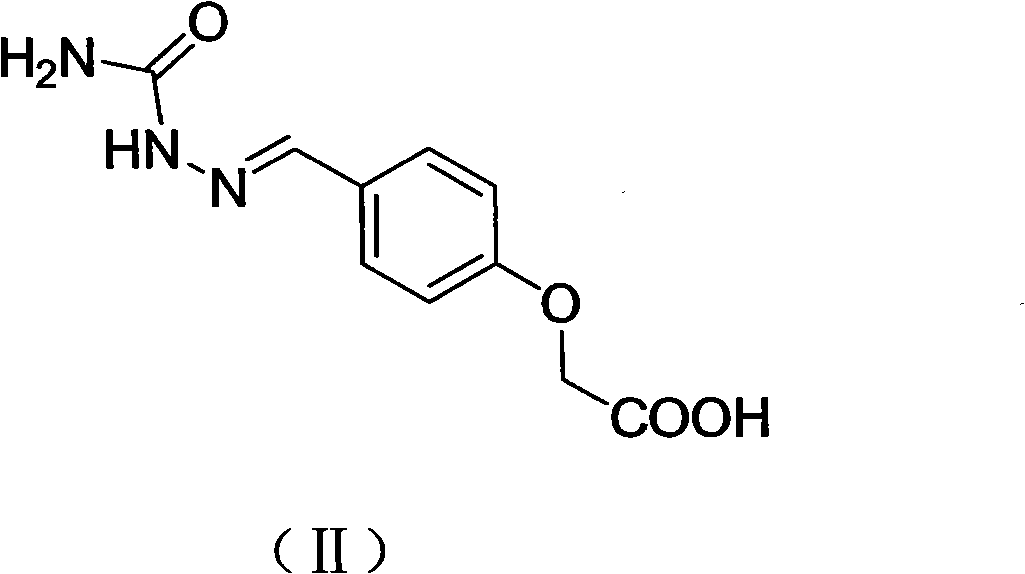
[0040] Add 1.06g of 2-(4-formylphenoxy)acetic acid into the round bottom flask, slowly add methanol until the 2-(4-formylphenoxy)acetic acid is completely dissolved, add 0.4g semicarbazide during stirring, and stir at room temperature After overnight, the reaction was completed, filtered, washed twice with water and twice with methanol, and dried to obtain 0.90 g of white powder, the structural formula of which was shown in formula (II). CEPSEM spectrum data: APCI-MSm / z: 237[M+H]+; 1H NMR (600MHz, d6-DMSO, TMS): δ10.09(s, 1H); 7.78(s, 1H); 7.64(d, J = 9.0 Hz, 2H); 6.91 (d, J = 9.0 Hz, 2H); 4.71 (s, 2H). The above spectral data can be assigned correctly, which is consistent with the CEPSEM structure, indicating that the hapten CEPSEM was synthesized successfully.
[0041]
Embodiment 3
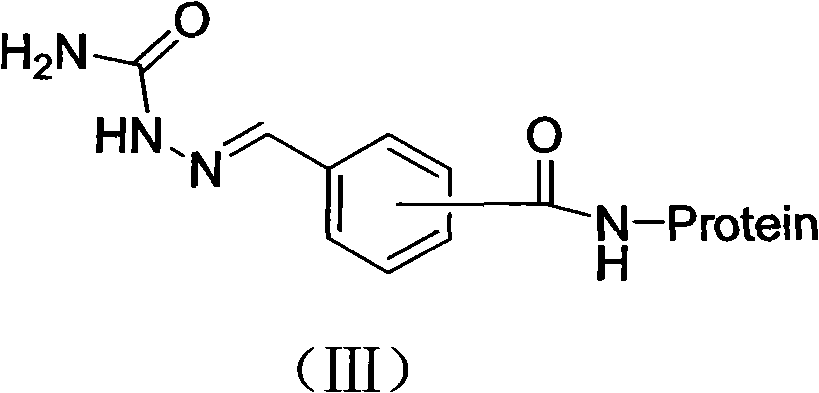
[0042] The preparation method of embodiment 3 immunogen CEPSEM-BSA
[0043] Take the hapten CEPSEM 0.1mmol and dissolve it in 2ml DMF, add DCC 27.5mg and NHS 14.4mg with stirring. Magnetically stir the reaction overnight at 4°C. After centrifugation, the supernatant night is liquid A; weigh 140 mg of BSA and dissolve it in 10 ml of PBS (pH 8.0) with a concentration of 0.1 mol / L, add 1 ml of DMF, stir and dissolve to prepare liquid B; under magnetic stirring , solution A was gradually dropped into solution B, and reacted at 4°C for 12 hours. After centrifugation, the supernatant was taken and dialyzed with normal saline at 4°C for 3 days, and the dialysate was changed 3 times a day. The obtained whole antigen was dispensed into 0.5ml centrifuge tubes at a concentration of 1 mg / ml, and frozen in a -20°C refrigerator for immunization.
[0044] The prepared semicarbazide derivative immunogen has a structure shown in formula (V):
[0045]
[0046] Immunogen identification: The c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com