5-methyl morpholine-3-amino-2-oxazolidinone derivative hapten, antigen and antibody, and application thereof
A technology of methylmorpholine and oxazolidinone, applied in the field of immunology, to achieve high titer, high sensitivity and stable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation method of 5-methylmorpholine-3-amino-2-oxazolidinone derivative hapten MCPAMOZ
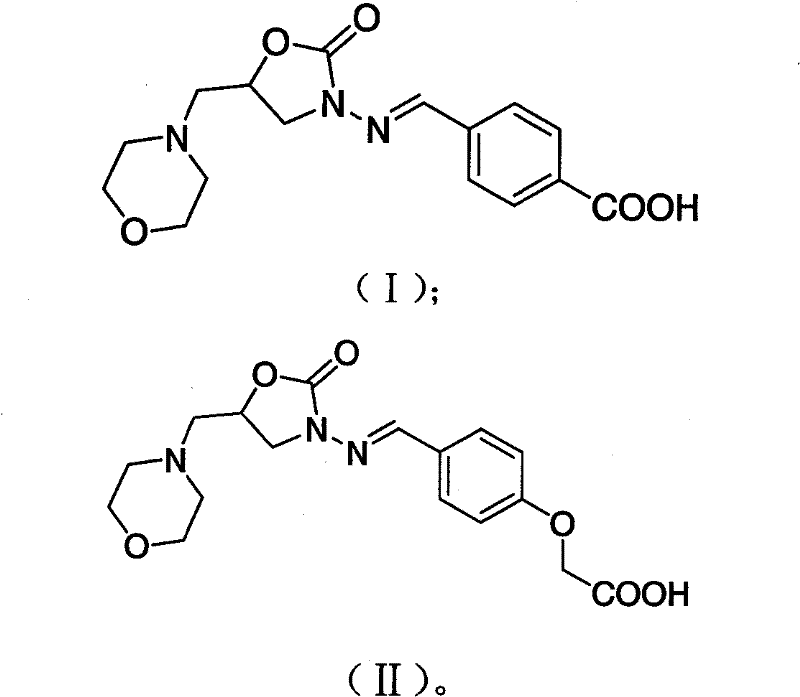
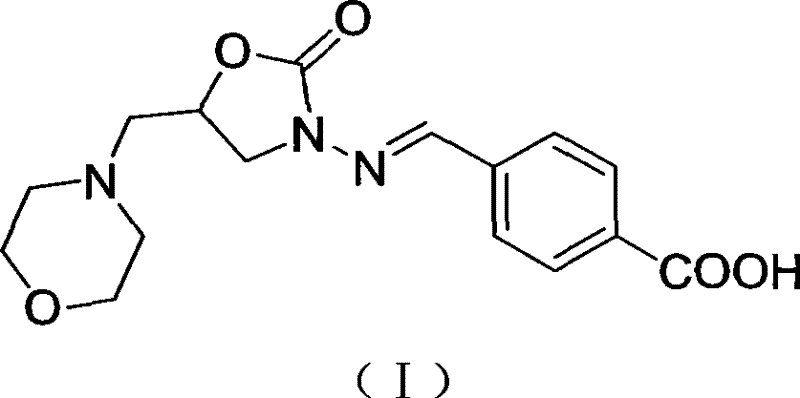
[0039] Add 1.15g of p-carboxybenzaldehyde into a 50ml round bottom flask, slowly add methanol until the p-carboxybenzaldehyde is completely dissolved, add 0.5g of 5-methylmorpholine-3-amino-2-oxazolidinone while stirring, and stir at room temperature overnight. Reaction finishes filtering, washes twice with water, washes twice with methanol, is dried, and obtains 0.80g white powder, and its structural formula is as shown in (I). ESI-MS (negative) m / z: 332 (M—H) - . 1 H NMR (400MHz, d 5 -Pyridine, TMS): δ 8.44 (d, J=8.3, 2H); 7.99 (d, J=8.3, 2H) 7.94 (s, 1H); 4.96 (qd, J 1 =6.2,J 2 =6.2,J 3 =6.3,J 4 = 8.4, 1H); 4.09 (t, J = 8.9Hz, 1H); 3.78 (dd, J1 = 6.7, J2 = 8.8, 1H); 3.69-3.64 (m, 4H); 2.67 (dd, J 1 =6.2,J 2 = 13.3, 1H); 2.61 (dd, J 1 =5.7,J 2 =13.4); 2.51-2.46 (m, 4H)
[0040]
[0041]
Embodiment 2
[0042] Example 2 Preparation method of 5-methylmorpholine-3-amino-2-oxazolidinone derivative hapten CEPAMOZ
[0043]Add 1.06g of 2-(4-formylphenoxy)acetic acid into the round bottom flask, slowly add methanol until p-carboxybenzaldehyde is completely dissolved, add 0.5g of 5-methylmorpholine-3-amino-2-oxazole while stirring Alkanone, stirred overnight at room temperature. After the reaction was completed, filtered, washed twice with water and twice with methanol, and dried to obtain 0.93 g of white powder, the structural formula of which was shown in formula (II). ESI-MS (negative) m / z: 362 (M-H) - . 1 H NMR (600MHz, d 5 -Pyridine, TMS): δ 7.89-7.87 (m.3H); 7.22 (d, J=8.8, 2H); 5.02 (s, 2H); 4.88 (qd, J 1 =6.31, J2=6.31, J3=6.35, J4=8.5, 1H); 4.01(t, J=8.8Hz, 1H); 3.70(dd, J1=6.8, J2=8.7, 1H); 3.67-3.65(m , 4H); 2.63(dd, J1=6.2, J2=13.3, 1H); 2.56(dd, J1=5.6, J2=13.4); 2.51-2.44(m, 4H)
[0044]
Embodiment 3
[0045] Example 3 Preparation method of immunogen CEPAMOZ-BSA
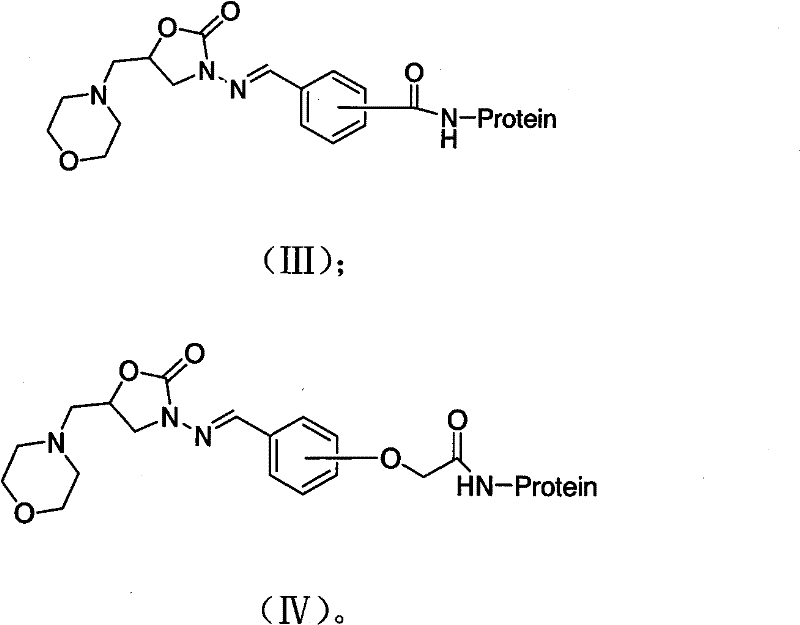
[0046] Dissolve 0.1mmol of the hapten CEPAMOZ in 2ml of DMF, stir and add 27.5mg of DCC and 14.4mg of NHS, and react overnight with magnetic stirring at 4°C. After centrifugation, the supernatant is liquid A; weigh 140mg of BSA and dissolve in 10ml with a concentration of 0.1mol / L Add 1ml of DMF to the PBS (pH8.0), stir and dissolve to prepare liquid B; under magnetic stirring, liquid A is gradually dropped into liquid B, and react at 4°C for 12 hours. After centrifugation, take the supernatant, dialyze with normal saline at 4°C for 3 days, change the dialysate 3 times a day, and distribute the whole antigen in 0.5ml at a concentration of 1mg / ml
[0047] Store in a centrifuge tube and freeze at -20°C for immunization. The structure of the prepared 5-methylmorpholine-3-amino-2-oxazolidinone derivative immunogen CEPAMOZ-BSA is shown in formula (V):
[0048]
[0049] Immunogen identification: The carrier protein ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com