Anti-EPHRINB2 antiboies and methods using same
A technology of antibodies and uses, applied in the field of molecular biology, can solve problems such as net balance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[1260] v. Preparation of antibody-drug conjugates
[1261]In the antibody-drug conjugate (ADC) of the present invention, the antibody (Ab) is conjugated to one or more drug moieties (D) via a linker (L), for example, about 1 to about 20 moieties per antibody. drug section. The ADC of general formula I can be prepared through several routes using organic chemical reactions, conditions and reagents known to those skilled in the art, including: (1) the nucleophilic group of the antibody reacts with a divalent linker reagent through a covalent bond to form Ab-L, which subsequently reacts with the drug moiety D; and (2) the nucleophilic group of the drug moiety reacts via a covalent bond with a divalent linker reagent to form D-L, which subsequently reacts with the nucleophilic group of the antibody. Additional methods for preparing ADCs are described herein.
[1262] Ab-(L-D)p I
[1263] A joint may consist of one or more joint components. Exemplary linker building blocks incl...
Embodiment 1
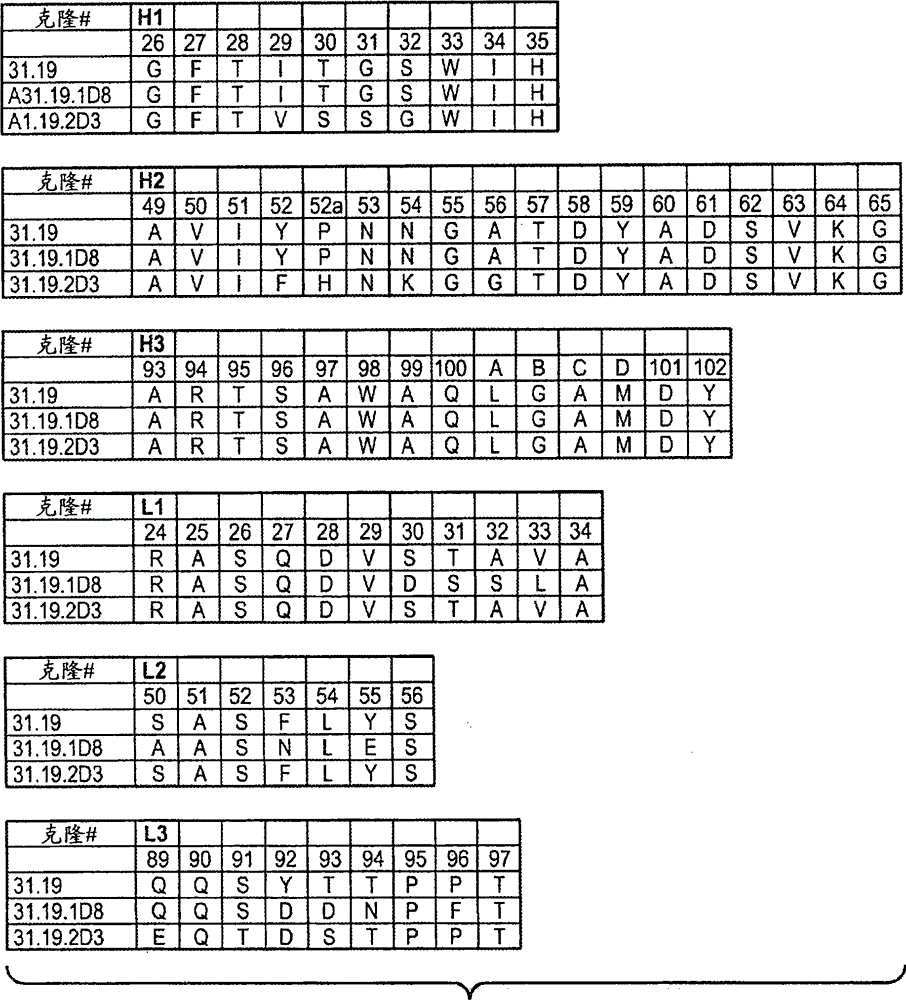
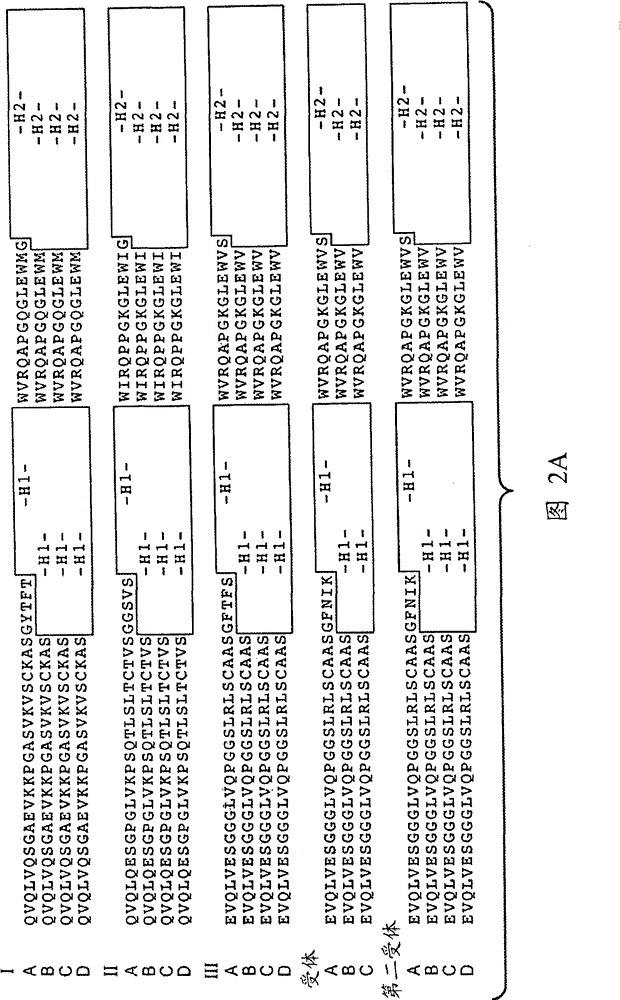
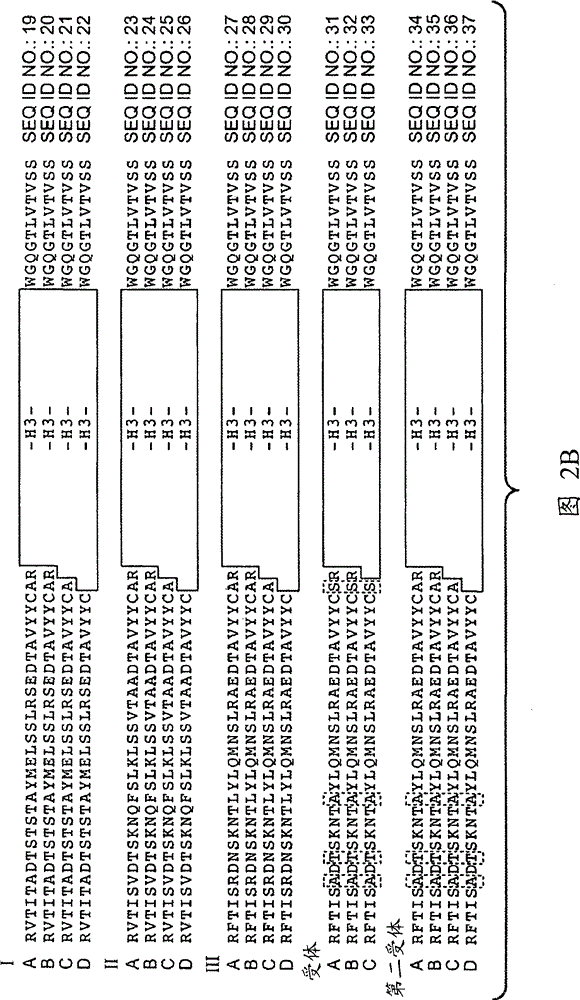
[1337] Example 1: Generation of Anti-EphrinB2 Antibodies
[1338]Various methods are known in the art for generating phage display libraries from which antibodies of interest can be obtained. A synthetic phage antibody library was constructed on a single framework (humanized anti-ErbB2 antibody, 4D5) by introducing diversity within the complementarity-determining regions (CDRs) of the heavy and light chains (Lee, C.V. et al. J Mol Biol 340, 1073 -93 (2004); Liang, W.C. et al. J Biol Chem 281, 951-61 (2006)). Plate panning of naive libraries against His-tagged human EphrinB2 immobilized on maxisorp immunoplates. After four rounds of enrichment, clones were randomly picked and specific binders were identified using phage ELISA. The resulting hEphrinB2-binding clones were further screened with His-tagged mouse EphrinB2 protein to identify cross-species clones. Clone 19 performed well in these assays and was selected for further characterization. For each positive phage clone,...
Embodiment 2
[1339] Example 2: Characterization of anti-EphrinB2 antibodies
[1340] To determine the binding affinity of mouse anti-EphrinB2 mAb, BIAcore TM -3000 (BIAcore, Inc., Piscataway, NJ) for surface plasmon resonance (SRP) measurements. Briefly, the carboxylate was activated with N-ethyl-N'-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) according to the supplier's instructions. Methylated dextran biosensor chip (CM5, BIAcore Inc.). The anti-EphrinB2 antibody was diluted to 5 μg / ml with 10 mM sodium acetate pH 4.8, and injected at a flow rate of 5 μl / min to obtain approximately 500 response units (RU) of the conjugated antibody. Next, 1M ethanolamine was injected to block unreacted groups. For kinetic measurements, two-fold serial dilutions of human or murine EphrinB2-His molecules in PBS containing 0.05% Tween-20 were injected at 25°C at a flow rate of approximately 25 μl / min. Association rates (k on ) and dissociation rate (k off ). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com