Method for producing cyclic ester-modified glucan derivative
A manufacturing method and glucan technology, applied in the field of molded products, can solve the problems of complicated processes, rising manufacturing costs, and damage to the fluidity of products, and achieve the effect of high grafting efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
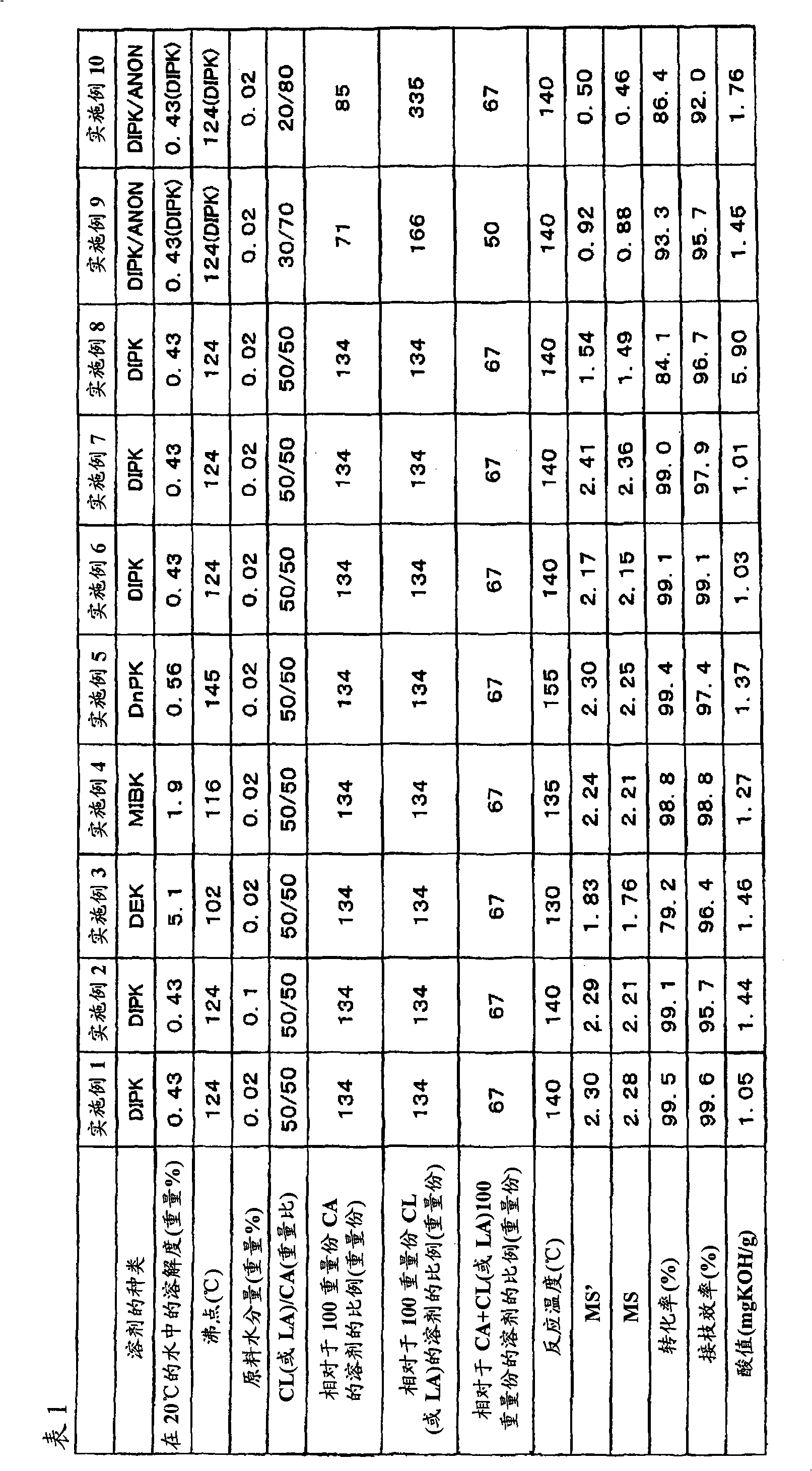
[0144] In a reactor with a stirrer and anchor-type stirring blades, add 50 parts of cellulose acetate (manufactured by Daicel Chemical Industry Co., Ltd., L-20, the average degree of substitution is 2.41, the molecular weight of each glucose unit 263.2, a specific gravity of 1.33, and an average degree of polymerization of 140), and dried under reduced pressure at 110° C. and 4 Torr (= about 530 Pa) for 4 hours. Then, the reactor was purified with dry nitrogen, a reflux condenser was installed, and 50 parts of ε-caprolactone and 67 parts of diisopropyl ketone (DIPK) that had been dried and distilled were added in advance, heated to 140 °C, stirred, and Cellulose acetate dissolves homogeneously. The moisture in the dissolved reaction solution was measured with a Karl Fischer moisture analyzer and found to be 0.02% by weight. 0.25 parts of monobutyltin trioctoate was added to this reaction liquid, and it heated at 140 degreeC, stirring for 2 hours. Then, the reaction solution ...
Embodiment 2
[0146]In the reactor with stirrer and anchor type stirring blade, add 50 parts of cellulose acetate (manufactured by Daicel Chemical Industry Co., Ltd., L-20, the average degree of substitution is 2.41, the molecular weight of each glucose unit is 263.2, The specific gravity is 1.33, and the average degree of polymerization is 140), and dried under reduced pressure at 110°C and 4 Torr for 4 hours. Then, the reactor was purified with dry nitrogen, a reflux condenser was installed, and 50 parts of ε-caprolactone and 67 parts of diisopropyl ketone (DIPK) that had been dried and distilled were added in advance, heated to 140 °C, stirred, and Cellulose acetate dissolves homogeneously. Here, water was added so that the water content in the dissolved reaction liquid was 0.1% by weight. 0.25 parts of monobutyltin trioctoate was added to this reaction liquid, and it heated at 140 degreeC, stirring for 2 hours. Then, the reaction solution was cooled to room temperature to terminate th...
Embodiment 3
[0148] In the reactor with stirrer and anchor type stirring blade, add 50 parts of cellulose acetate (manufactured by Daicel Chemical Industry Co., Ltd., L-20, the average degree of substitution is 2.41, the molecular weight of each glucose unit is 263.2, The specific gravity is 1.33, and the average degree of polymerization is 140), and dried under reduced pressure at 110°C and 4 Torr for 4 hours. Then, the reactor is purified with dry nitrogen, and a reflux condenser is installed, and 50 parts of ε-caprolactone and 67 parts of diethyl ketone (DEK) that have been dried and distilled in advance are added, heated to 125 ° C, stirred, and the fiber Acetate dissolves evenly. The moisture in the dissolved reaction solution was measured with a Karl Fischer moisture analyzer and found to be 0.02% by weight. 0.25 parts of monobutyltin trioctoate was added to this reaction liquid, and it heated at 125 degreeC, stirring for 3 hours. Then, the reaction solution was cooled to room temp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com