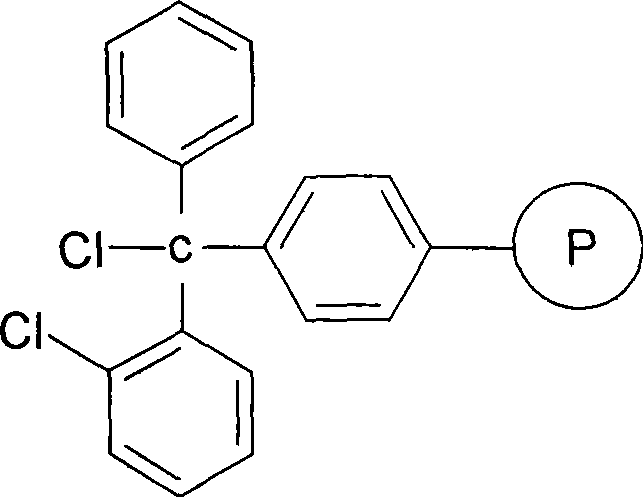
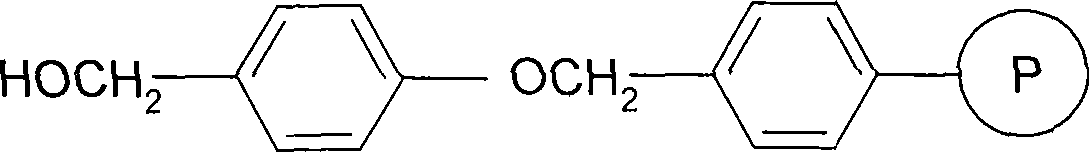Solid phase synthesis method for thymosin beta 4
A solid-phase synthesis, thymosin technology, applied in the field of biochemistry, can solve the problems of difficulty in purification, affecting the condensation rate of amino acids, increasing the probability of amino acid racemization, etc., achieving the effects of less impurities, shortening synthesis time, and easy separation and purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of Fmoc-Ser (tBu)-king resin
[0042] Operation: (1) Put 12.5g (5mmol) of King Resin (Tianjin Nankai Hecheng Technology Co., Ltd.) in a peptide synthesis reactor, wash with N,N-dimethylformamide (DMF), add DMF to swell for 30min Afterwards, 3.8g of Fmoc-Ser(tBu)-OH was dissolved in 50ml of DMF and added to the reactor, 3.2ml of pyridine was added, and 2.8ml of 2,6-dichlorobenzoyl chloride was added dropwise. After the dropwise addition, the reaction was carried out at room temperature After 2 hours, wash three times with DMF, once with methanol, three times with dichloromethane, three times with methanol, and dry to obtain 14.3 g of Fmoc-Ser(tBu)-King resin.
Embodiment 2
[0043] Example 2: Fmoc-Thr(tBu)-Ile-Glu(OtBu)-Gln(Trt)-Glu(OtBu)-Lys(Boc)-Gln(Trt)-Ala-Gly-Glu(OtBu)-Ser(tBu )-Wang resin (A peptide fully protected resin) preparation
[0044] Operation: (1) Put 14.3g of Fmoc-Ser(tBu)-King resin in the polypeptide synthesis reactor, wash with 80ml of dichloromethane and methanol for 2 times alternately, 2 minutes each time, and drain. Add 80 ml of 20% piperidine / DMF solution, stir at room temperature for 20 minutes, and drain. The resin was alternately washed 3 times with 80 ml of dichloromethane and methanol for 2 minutes each time, and then drained.
[0045] (2) 8.0 g of Fmoc protected amino acids and O-benzotriazole-N, N, N', N'-tetramethyluronium tetrafluoroborate (TBTU, Shanghai Yanchang Biochemical Technology Development Co., Ltd.) were used in 50 ml After the DMF solution was dissolved, 4.4 ml of N,N-diisopropylethylamine was added, mixed evenly, added to the resin, and stirred at room temperature for 3 hours.
[0046] (3) Drain. T...
Embodiment 3
[0051] Embodiment 3: Preparation of Fmoc-Glu(OtBu)-2-chlororityl chloride resin
[0052]Operation: 100g (120mmol) of 2-chlororityl chloride resin was added to a 1000ml solid-phase synthesis reactor, 204g of Fmoc-Glu(OtBu)-OH was dissolved in 600ml of dichloromethane, and N,N-diisopropylethylamine ( DIEA) 167ml, stirred and mixed evenly, added to the solid-phase synthesis reactor, and reacted at room temperature for 1 hour. Drain. The resin was alternately washed 3 times with 800 ml of dichloromethane and methanol, 2 minutes each time, and then drained. 143.7 g of Fmoc-Glu(OtBu)-2-chlorotrityl chloride resin was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com