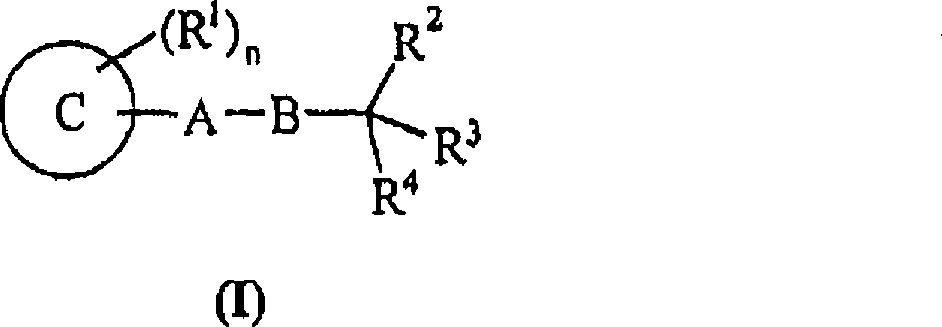
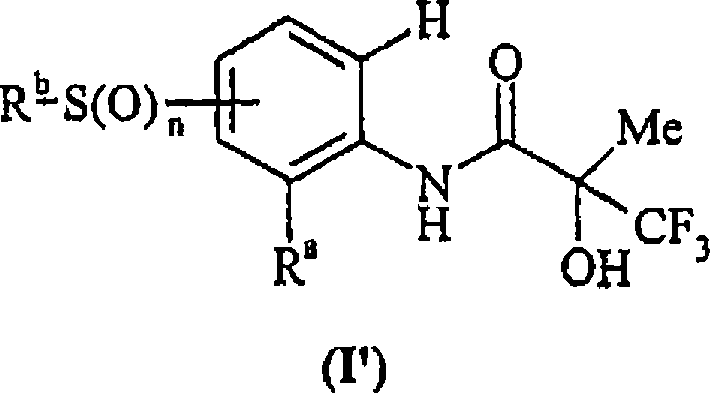
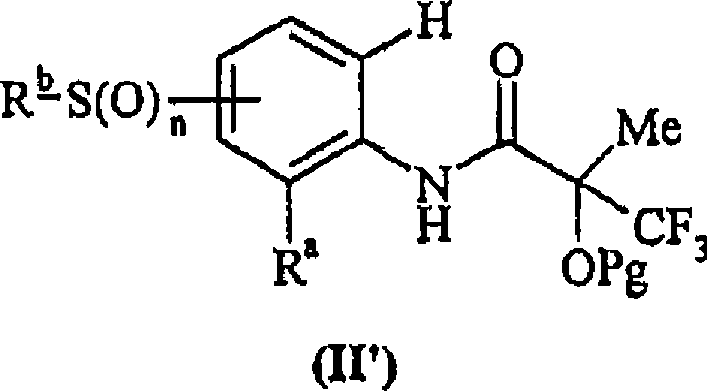
Use of compounds for the elevation of pyruvate dehydrogenase activity
A compound and active technology, applied in the application field of compounds in enhancing the activity of pyruvate dehydrogenase, can solve the problem of increased availability of lactic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0746] (R)-N-[2-chloro-4-(2-methylthiophenylsulfonyl)phenyl]-2-hydroxy-2-methyl-3,3, 3-Trifluoropropionamide
[0747] In (R)-N-[2-chloro-4-(2-fluorobenzenesulfonyl)phenyl]-2-hydroxy-2-methyl-3,3,3-trifluoropropionamide (Method 63) ( Sodium methyl mercaptan (49.5 mg) was added to a solution of 0.15 g) in NMP (1.5 ml), the resulting mixture was heated at 120°C for 18 nitric acid, and then cooled. A saturated aqueous solution of ammonium chloride (15ml) was added, and the mixture was extracted with ethyl acetate (2 x 50ml). The organic extracts were combined, washed with brine and dried. The volatile substances were removed by evaporation, and the residue was purified by chromatography on a silica gel Mega Bond E1ur column using 0-20% ethyl acetate / hexane to obtain the title compound as a solid (0.10 g). NMR: (CDCl 3 ): 1.75(s, 3H), 2.4(s, 3H), 3.6(brs, 1H), 7.3(t, 1H), 7.35(t, 1H), 7.55(m, 1H), 7.9(dd, 1H) , 8.05 (d, 1H), 8.25 (dd, 1H), 8.6 (d, 1H), 9.25 (brs, 1H); MS (ESP - ): 45...
Embodiment 2-12
[0749] According to the method of Example 1, the following compounds were prepared using appropriate starting materials.
[0750] Example Compound NMR(CDCl 3 ) MS SM 2
[0751] 8
[0752] 1 Add three equivalents of sodium methyl mercaptan.
Embodiment 13
[0754] (R)-N-{2-chloro-4-[2-(methylsulfinyl)benzenesulfonyl]phenyl}-2-hydroxy-2-methyl -3,3,3-Trifluoropropionamide
[0755] In (R)-N-[2-chloro-4-(2-methylthiobenzenesulfonyl)phenyl]-2-hydroxy-2-methyl-3,3,3-trifluoropropionamide (Example 1) Add m-chloroperbenzoic acid (50%, 0.293g) to a solution of (0.384g) in DCM (40ml). Stir the mixture at room temperature for 6 hours, and then use saturated aqueous sodium bicarbonate (3 x 100ml), water ( 100ml) and brine, and then dried. The volatiles were removed by evaporation, and the residue was purified by chromatography on a silica gel Mega Bond Elur column, eluting with 50-70% ethyl acetate / hexane to obtain the title compound as a solid (0.26 g). Mp 118-120℃; NMR(CDCl 3 ): 1.70 (s, 3H), 3.0 (m, 3H), 4.85 (brs, 1H), 7.75 (t, IH), 7.85 (m, 2H), 8.0 (m, 1H), 8.15 (d, 1H) , 8.3 (d, 1H), 8.65 (dd, 1H), 9.40 (brs, 1H); MS (ESP - ): 468; EA: Measured value: C, 44.3; H, 3.7; N, 2.6%; C 17 H 16 ClF 3 NO 5 S 2 ·0.125 C 4 H 8 O 2 ·0.3C 4 H 10 Ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com