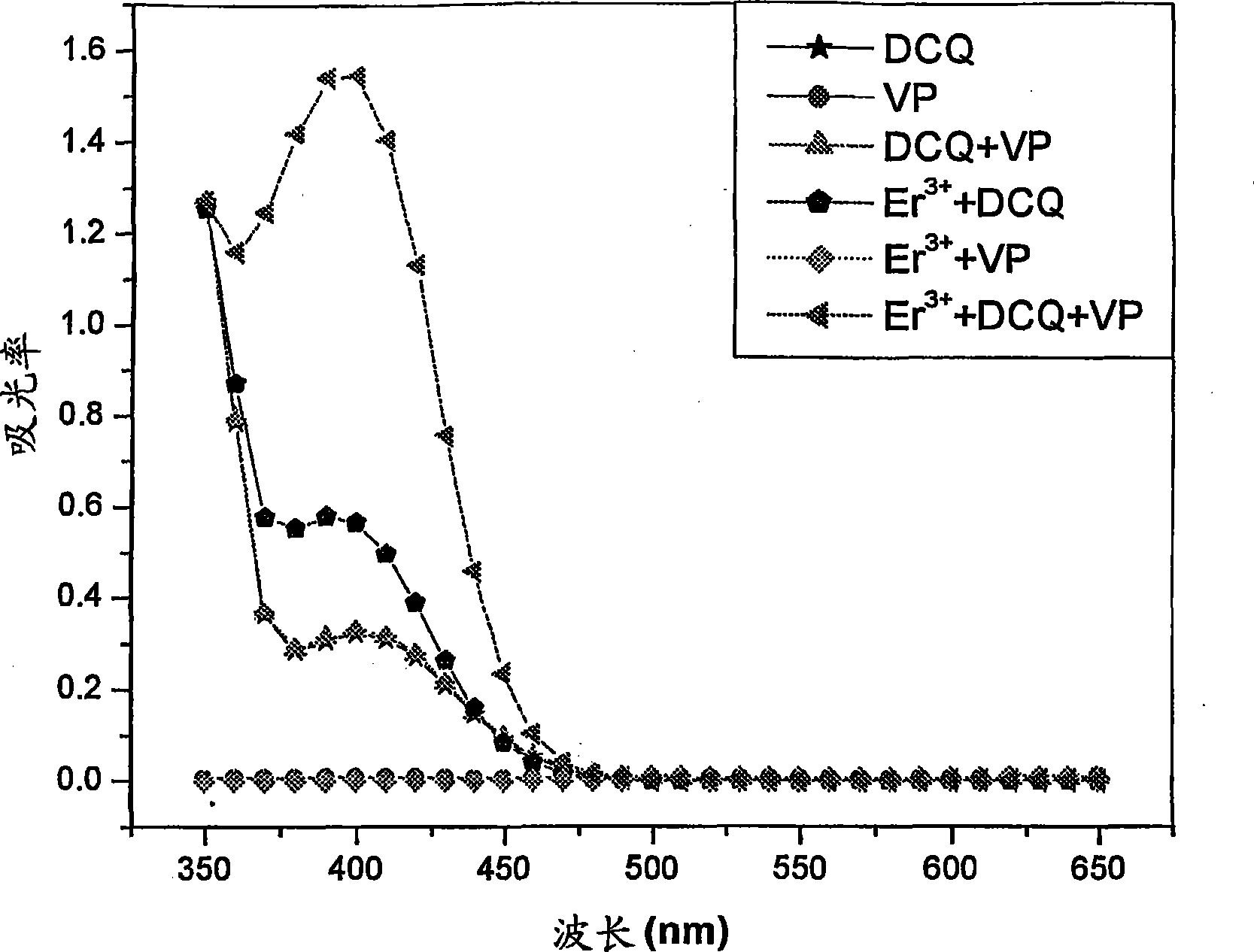
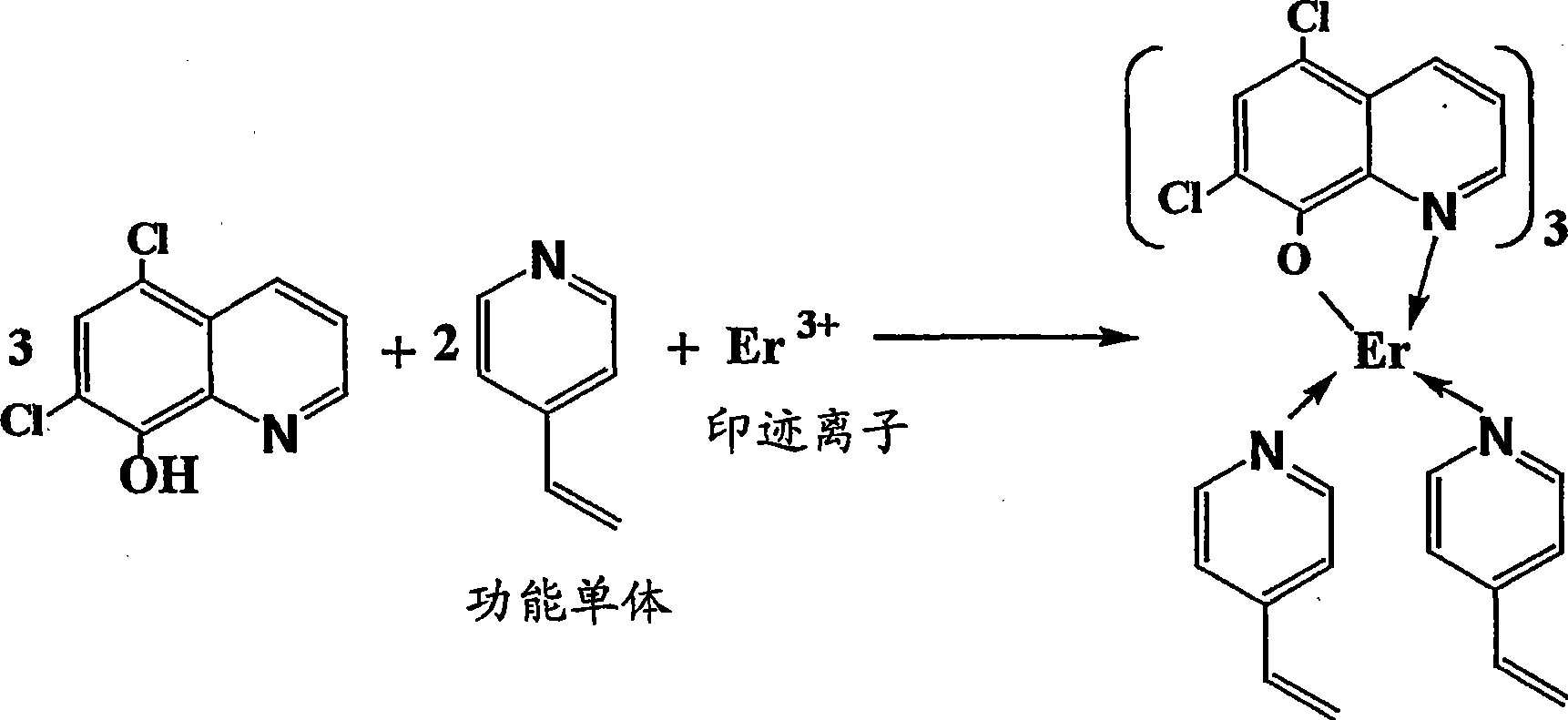
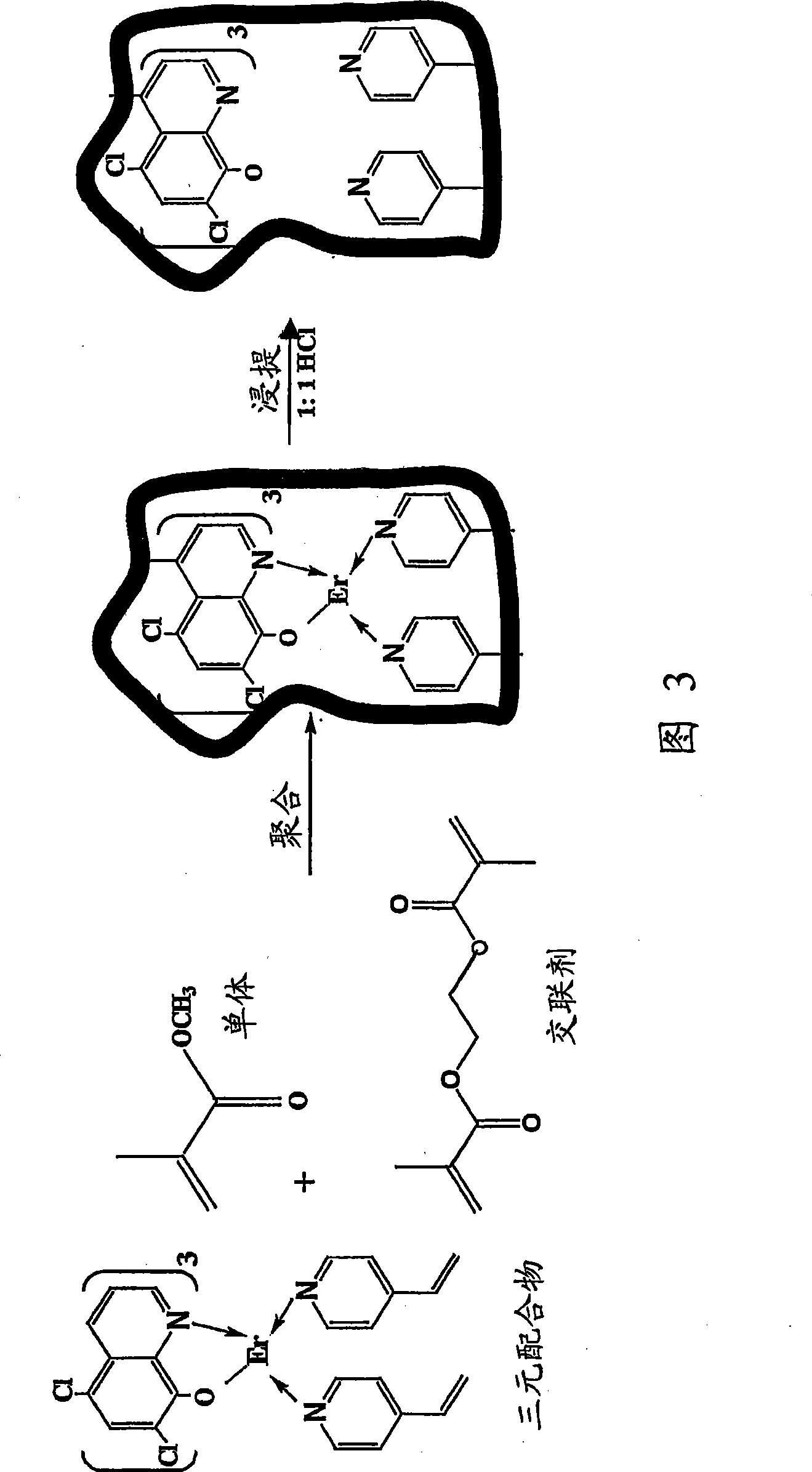
Synthesis of ion imprinted polymer particle
A technology of ion imprinting and polymers, applied in other chemical processes, chemical instruments and methods, etc., can solve the problems of uncommonly used separation and connection of inorganic ions, and separation not involved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: γ-radiation polymerization
[0062] In a 50 ml round bottom flask, 1.0 mM erbium chloride (0.44 g), 3.0 mM DCQ (0.64 g) and 2 mM VP (0.21 g) were added and dissolved in 5 or 10 ml 2-methoxyethanol under stirring. Add 4 (0.4g) or 8 (0.8g) and 12 (1.2g) mM MMA and 16 (3.17g) or 32 (6.34g) and 48 (9.52g) mM EGDMA and stir until a homogeneous solution is obtained. Transfer the monomer mixture to a test tube, cool to 0°C, use N 2 Flush for 10 minutes and seal.
[0063] Application Co 60 Source, these solutions were irradiated for 4 hours under 1M Rad gamma radiation. The formed solid was washed with water and dried in an oven at 50°C. 5.70, 9.43 and 14.27g polymer materials with 4, 8 and 12mM functional monomers were obtained, respectively. The erbium ion-intercalated polymer was leached with 50% (v / v) HCl while stirring for 6 hours. After drying in an oven at 50°C, 4.14, 7.52 and 11.29g polymer materials with 4, 8 and 12mM functional monomers were obtained.
Embodiment 2
[0064] Example 2: Photochemical polymerization
[0065] In a 50 ml round bottom flask, 1.0 mM erbium chloride (0.44 g), 3.0 mM DCQ (0.64 g) and 2.0 mM VP (0.21 g) were added, and dissolved in 10 ml 2-methoxyethanol under stirring. Add 8mM MMA (0.8g), 32mM EGDMA (6.35g) and 50mg AIBN and stir until a homogeneous solution is obtained. Transfer the monomer mixture to a test tube, cool to 0°C, use N 2 Flush for 10 minutes and seal. These solutions were polymerized by UV radiation (300 nm) for 4, 8 and 16 hours. The solid formed was washed with water and dried in an oven at 50°C. 7.55, 9.85 and 9.95 g of polymer material were obtained with UV radiation (300 nm) for 4, 8 and 16 h. The erbium ion-intercalated polymer was leached with 50% (v / v) HCl while stirring for 6 hours. After drying in an oven at 50°C, 5.35, 7.31, and 7.36 g of polymer materials were irradiated with UV for 4, 8, and 16 hours, respectively.
Embodiment 3
[0066] Example 3: Thermal polymerization
[0067] In a 50 ml round bottom flask, 1.0 mM erbium chloride (0.44 g), 3.0 mM DCQ (0.64 g) and 2.0 mM VP (0.21 g) were added, and dissolved in 10 ml 2-methoxyethanol under stirring. Add 8.0 mM MMA (0.8 g), 8, 16 and 32 mM EGDMA (1.59, 3.17 and 6.34 g) and 50 mg AIBN, and stir until a homogeneous solution is obtained. Cool the polymerization mixture to 0°C and use N 2 Flush for 10 minutes, seal and heat and stir in an oil bath at about 80°C for 2 hours. The solid formed was washed with water and dried in an oven at 50°C. 4.32, 5.50 and 8.84 g of polymer material with 50%, 66% and 80% crosslinking monomer were obtained. The polymer embedded with erbium ions was leached with 100 ml 50% (v / v) HCl, stirred for 6 hours, filtered, and dried in an oven at 50°C. Obtained 2.59, 3.90 and 7.90 g of erbium ion imprinted polymer materials.
[0068] Advantages of the invention:
[0069] The liquid-liquid extraction method replaces the conventional ion e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com