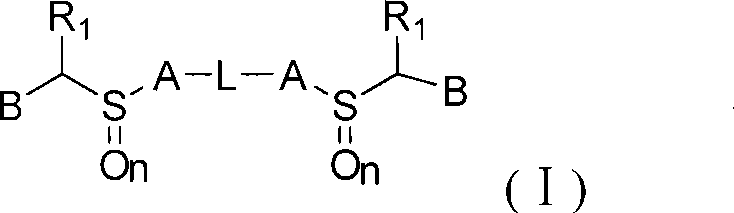
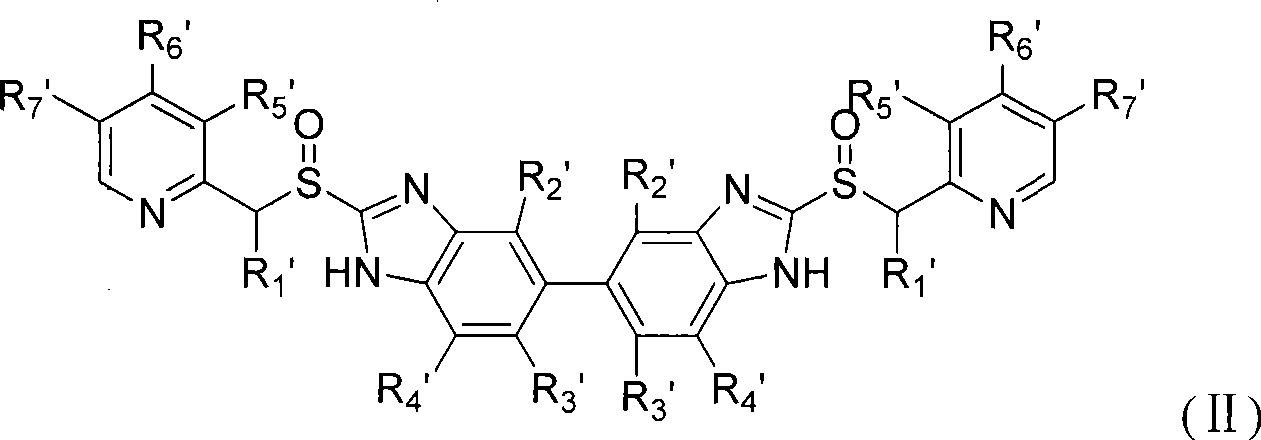
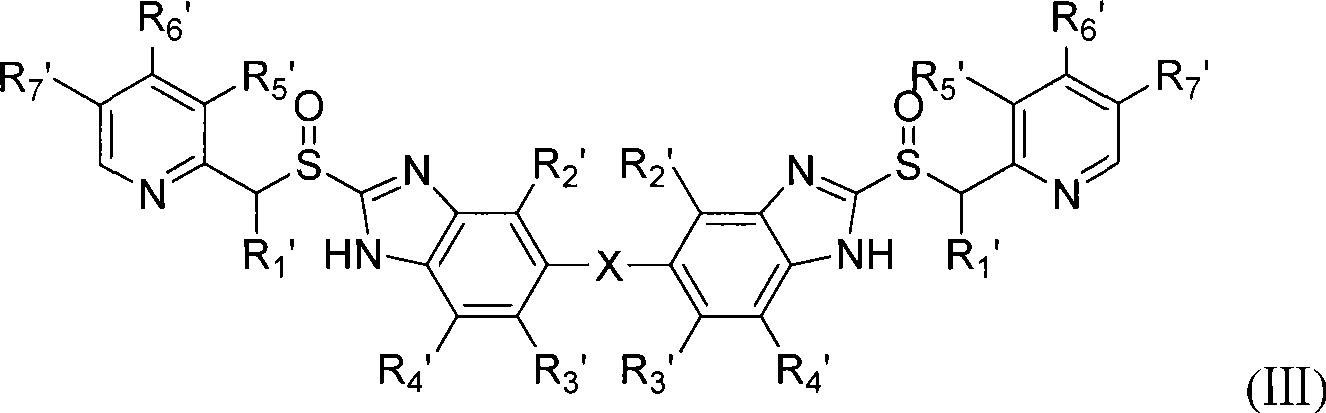
Novel benzimidazoles compounds
A technology of benzimidazoles and compounds, applied in the field of bisbenzimidazoles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0172] Example 1: 5,5'-bis-[2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylthio]-1H-benzimidazole] (Compound 1 ) preparation
[0173]Add 5,5'-bis-(2-mercapto-1H-benzimidazole) (1.0 g), absolute ethanol (15 ml), sodium hydroxide (0.84 g) in water (20 ml) to the reaction flask in sequence , stirred at room temperature for 30 minutes, and the solids were completely dissolved. 2-Chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride (1.5 g) was added, and the mixture was refluxed for 2 hours. The solvent was distilled off under reduced pressure, the obtained oil was added with water to precipitate a solid, the pH was adjusted to 6 with glacial acetic acid, the solid was filtered off, dried, and purified by column chromatography (chloroform:methanol=90:1) to obtain 1.6 g of compound 1 with a yield of 80 %. 1 H-NMR (300MHz, DMSO-d 6 , ppm): 12.68 (br s, 1H), 8.19 (s, 1H), 7.67 (s, 1H), 7.50 (d, J=8.3, 1H), 7.42 (dd, J=8.3, J=1.5, 1H ), 4.71(s, 2H), 3.72(s, 3H), 2.30(s, 3H), 2....
Embodiment 2
[0174] Example 2: Preparation of 5,5'-bis-[2-[(3,4-dimethoxypyridin-2-yl)methylthio]-1H-benzimidazole] (compound 2)
[0175] Using the method of Example 1, 5,5'-bis-(2-mercapto-1H-benzimidazole) (1.0 g) and 2-chloromethyl-3,4-dimethoxypyridine hydrochloride (1.5 g) reaction to obtain 1.8 g of compound 2, 89%. 1 H-NMR (300MHz, DMSO-d 6 , ppm): 12.68 (s, 1H), 8.17 (d, J=5.7, 1H), 7.40-7.70 (m, 3H), 7.09 (d, J=5.7, 1H), 4.69 (s, 2H), 3.89 (s,3H), 3.82(s,3H).
Embodiment 3
[0176] Example 3: 5,5'-bis-[2-[[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylthio]-1H- The preparation of benzimidazole] (compound 3)
[0177] Using the method of Example 1, 5,5'-bis-(2-mercapto-1H-benzimidazole) (1.0 g) and 2-chloromethyl-3-methyl-4-(2,2,2- Trifluoroethoxy)pyridine hydrochloride (1.9 g) was reacted to obtain 1.9 g of compound 3, 80%. 1 H-NMR (300MHz, DMSO-d 6 , ppm): 12.68 (s, 1H), 8.31 (d, J=5.7, 1H), 7.40-7.80 (m, 3H), 7.09 (d, J=5.7, 1H), 4.90 (q, J=8.7, 2H), 4.75(s, 2H), 2.26(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com