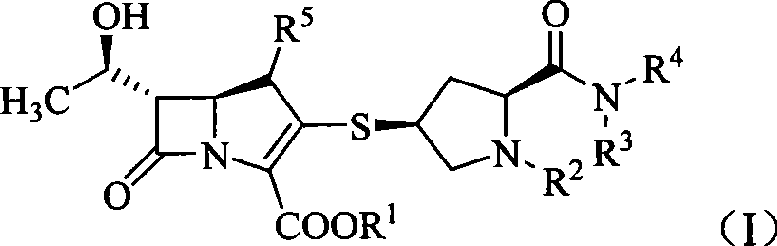
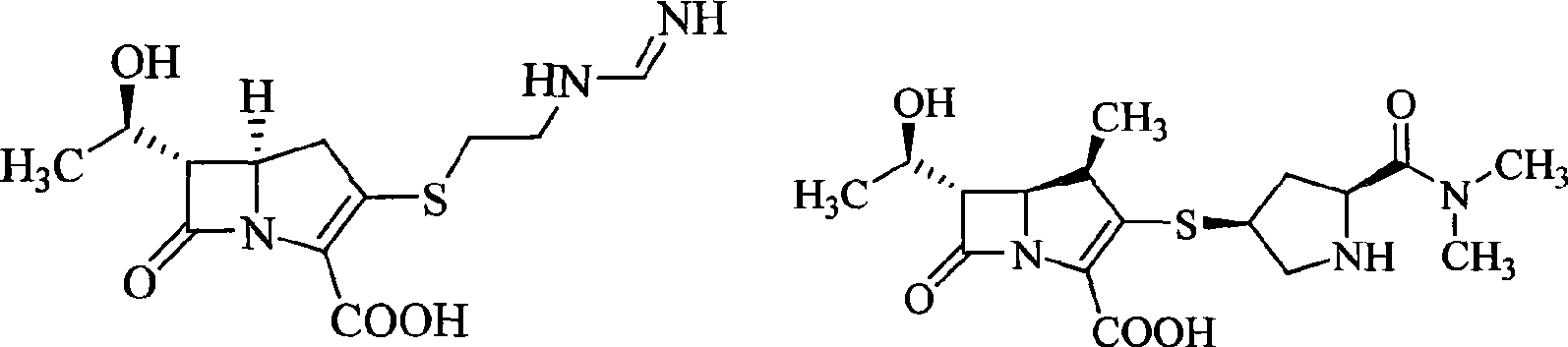
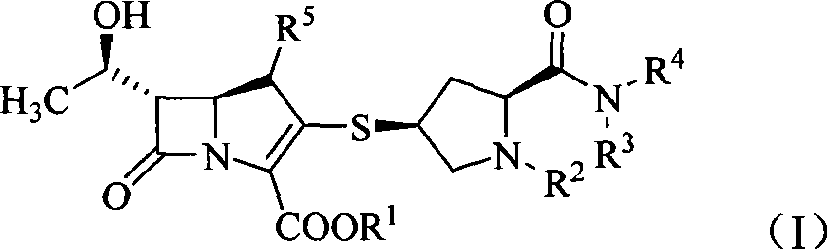
Carbapenem compound
A compound and hydrate technology, applied in the field of medicine, can solve the problems of not meeting clinical needs, weak antibacterial activity of MRSA, and low clinical utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1 (2S, 4S)-4-acetylthio-2-formyl [(2-tert-butylamino-acetyl-1-yl)amino]-1-(tert-butoxycarbonyl)pyridine Preparation of rolidine
[0095] Add (2S, 4S)-4-acetylthio-2-carboxy-1-(tert-butoxycarbonyl)pyrrolidine 8.7g (30mmol) and 100ml of anhydrous tetrahydrofuran to the dry reaction flask, under nitrogen protection, in Add 6.5g (40mmol) of 1,1-carbonyldiimidazole at room temperature, react for 0.5h, add (2-tert-butylamino)acetamide 7.8g (60mmol) in 100ml of tetrahydrofuran solution below 0°C, continue the reaction for 0.5h, then Add 40ml of 1mol / L hydrochloric acid dropwise, extract with ethyl acetate (50ml×2), wash the organic phase with water and saturated sodium chloride solution successively, concentrate under reduced pressure, and recrystallize the solid from a mixed solution of acetonitrile and ethyl acetate to obtain 10.4g , Yield: 86.5%.
Embodiment 2
[0096] Example 2 (2S, 4S)-4-mercapto-2-formyl [(2-(N-tert-butoxycarbonyl-tert-butylamino)-acetyl-1-yl)amino]-1-(tert Preparation of butoxycarbonyl)pyrrolidine
[0097] Add (2S,4S)-4-acetylthio-2-formyl[(2-tert-butylamino-acetyl-1-yl)amino]-1-(tert-butoxycarbonyl)pyrrolidine 10g into the reaction flask (25mmol) of dichloromethane solution in 80ml, cooled in an ice bath to 5°C, added 10ml of triethylamine, stirred for 5min, then added dropwise 10g of (Boc) 2 100ml of dichloromethane solution of O, stirred for 1h, added 100ml of water under ice-water cooling, separated the water layer, and extracted the water layer with 50ml×3 dichloromethane, combined the organic layers, dried over anhydrous sodium sulfate, and concentrated to dryness. Add 100ml of 2mol / L hydrochloric acid to the residue, stir for 2h, adjust to basicity with dilute alkaline solution, and precipitate a solid, which is recrystallized with an equal volume of acetonitrile and acetone mixed solution to obtain 9.4...
Embodiment 3
[0098] Example 3 (4R, 5S, 6S)-3-[(2S, 4S)-4-mercapto-2-formyl[(2-(N-tert-butoxycarbonyl-tert-butylamino)-acetyl-1- base) Amino]-1-(tert-butoxycarbonyl)pyrrolidin-4-yl]thio-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo- [3, 2, 0] G Preparation of -2-ene-2-carboxylic acid p-nitrobenzyl ester
[0099]In a dry reaction flask, add (4R, 5S, 6S)-3-diphenoxyphosphoryloxy-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1- Azabicyclo-[3,2,0]hept-2-ene-2-carboxylic acid p-nitrobenzyl ester 11.9g (20mmol) in 120ml of acetonitrile solution, cooled to below -10°C, add diisopropylethylamine 5ml and (2S,4S)-4-acetylthio-2-formyl[(2-(N-tert-butoxycarbonyl-tert-butylamino)-acetyl-1-yl)amino]-1-(tert-butyl Oxycarbonyl)pyrrolidine 10.1g (22mmol) in acetonitrile solution 80ml, stirred at 0°C for 15h. After the reaction was completed, 300 ml of ethyl acetate was added to dilute, washed with water and saturated brine successively, the organic layer was dried and concentrated to obtain 10.4 g o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com