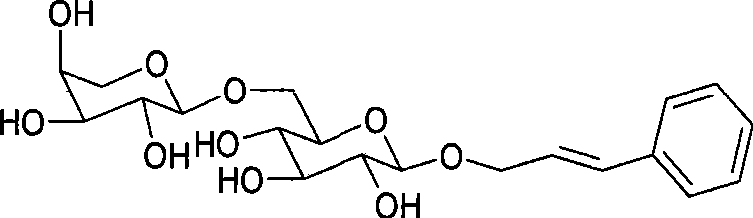
Method for preparing activity constituent rosavin in rhodiola rosea
An active ingredient, rose red technology, applied in the field of synthesis of active ingredients of Rhodiola rosea, can solve the problems of unsuitable mass production, high cost, and no reports on the chemical synthesis of rosavin.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
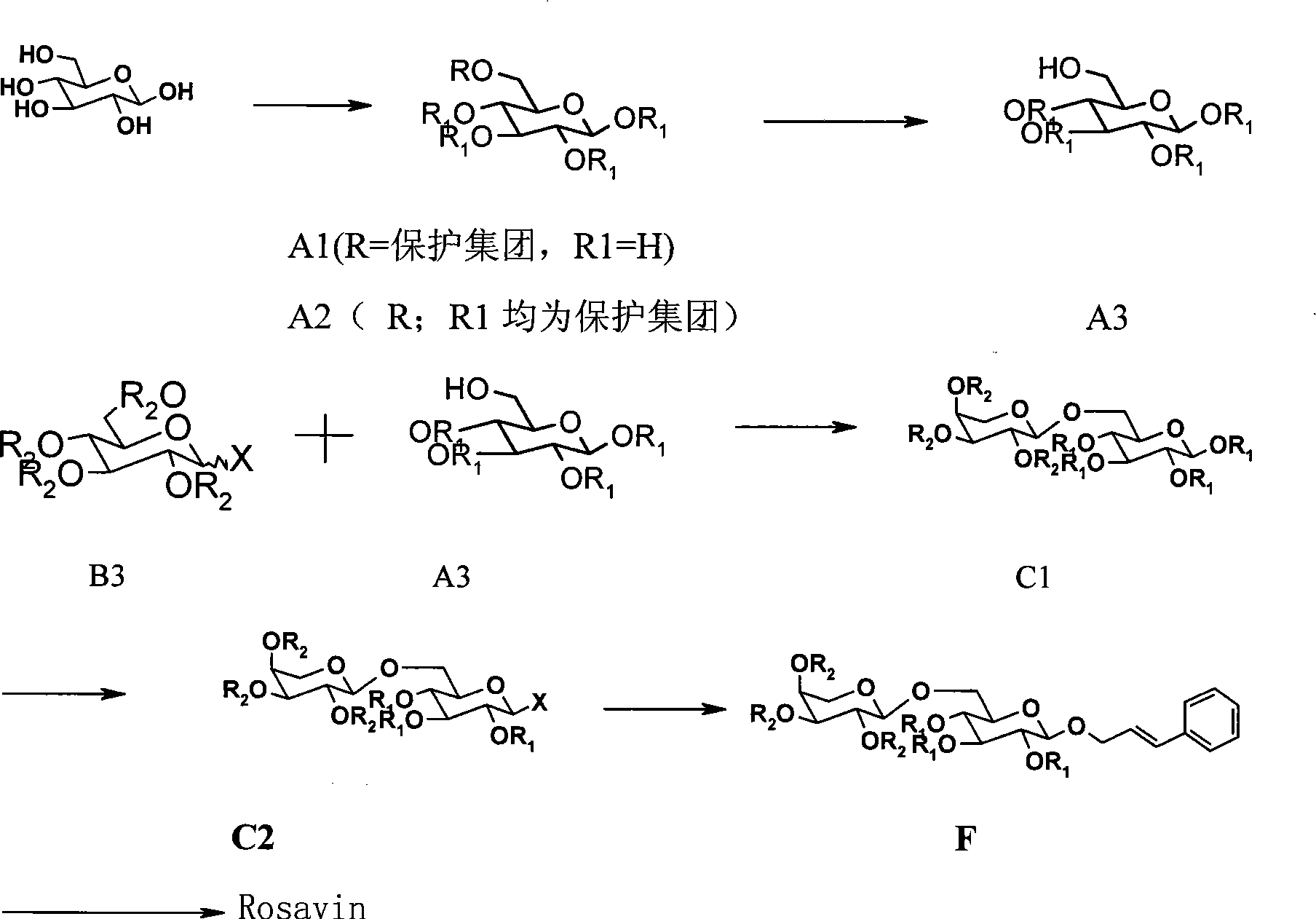
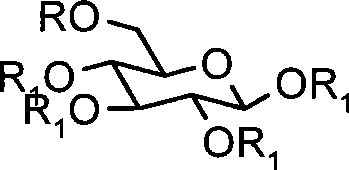
[0023] Example 1 Synthesis of 6-O-triphenylmethyl-glucose (A1)
[0024]
[0025] (R is Tr R1 is H)
[0026] Dissolve D-glucose (180.16g, 1.0mol) in pyridine (1800ml), add TrCl (362g, 1.3mol) and DMAP (1.833g, 0.015mol), stir overnight at 80°C, TLC (ethyl acetate: methanol = 8: 1) The detection reaction is complete, and it is directly used for the next reaction.
Embodiment 2
[0027] Example 2 Synthesis of 1,2,3,4-tetra-O-benzoyl-6-O-triphenylmethyl-D-glucopyranose (A2)
[0028] Add benzoyl chloride (521ml) dropwise to the solution of compound A1 in an ice-water bath, stir overnight at room temperature, TLC (petroleum ether: ethyl acetate = 4:1) detects that the reaction is complete, add methanol (140.86ml) dropwise to quench the reaction, and use Ethyl acetate (4000ml) was diluted, followed by 3% HCl solution (1500ml*4), saturated NaHCO 3 The solution (1500ml*1) and saturated brine (1500ml*2) were washed, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain about 900g of a brown syrupy product. .
Embodiment 3
[0029] Example 3 Synthesis of 1,2,3,4-tetra-O-benzoyl-D-glucopyranose (A3)
[0030] Dissolve A2 (900g) in dichloromethane (2000ml), add methanol (1000ml), add p-toluenesulfonic acid to adjust the pH of the reaction solution to 2, stir at room temperature overnight, and detect by TLC (petroleum ether: ethyl acetate = 4:1) After the reaction was complete, triethylamine was added to adjust the pH of the reaction solution to 7, concentrated, and column chromatography (eluent: petroleum ether: ethyl acetate = 4:1) gave 381.8 g of a light yellow solid, HPLC: 87.73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com