Process for methane conversion
A methane, conversion technology, applied in organic chemistry, hydrocarbons, bulk chemical production, etc., can solve problems such as high exothermic carbon oxides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
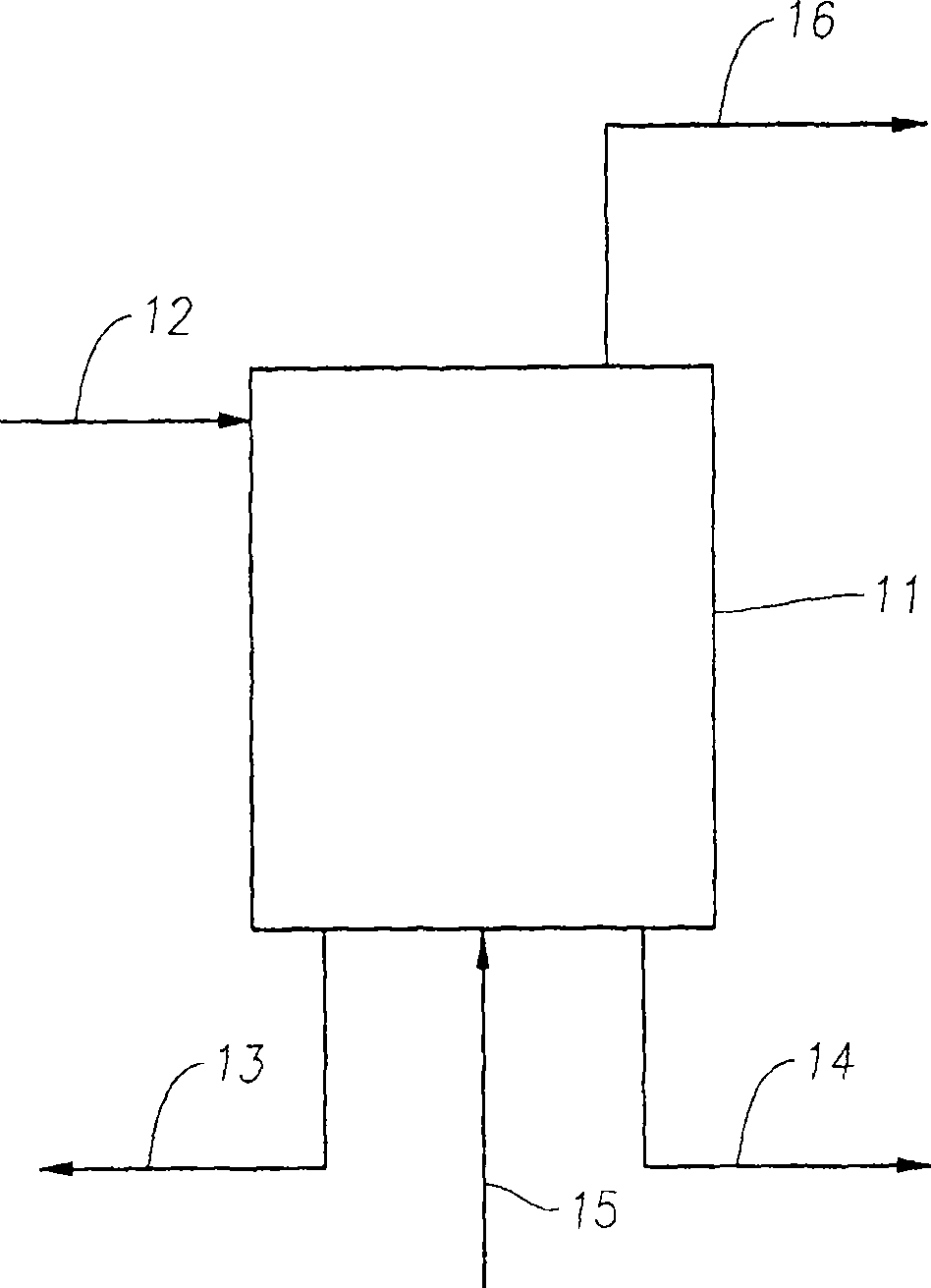
[0224] The Mo / ZSM-5 catalyst was prepared by NH 4 The required amount of ammonium heptamolybdate (ammonium heptamolybdate) solution (Si / Al) was impregnated on the ZSM-5 carrier 2 Ratio 28) by incipient wetness followed by drying at 120°C for 2 hours and then final calcination at 500°C for 6 hours in flowing air. A nominal molybdenum loading of 2.7 wt.% (wt.% metal based on total catalyst weight) was targeted; although secondary variables in molybdenum loading did not affect the conclusions obtained. Each Mo / ZSM-5 catalyst sample (after calcination) was pelletized, crushed, and sieved to a 30-60 mesh particle size. Catalyst tests of the Mo / ZSM-5 catalyst were carried out in a quartz reactor, which was filled to form a fixed-bed using a quartz wool support.
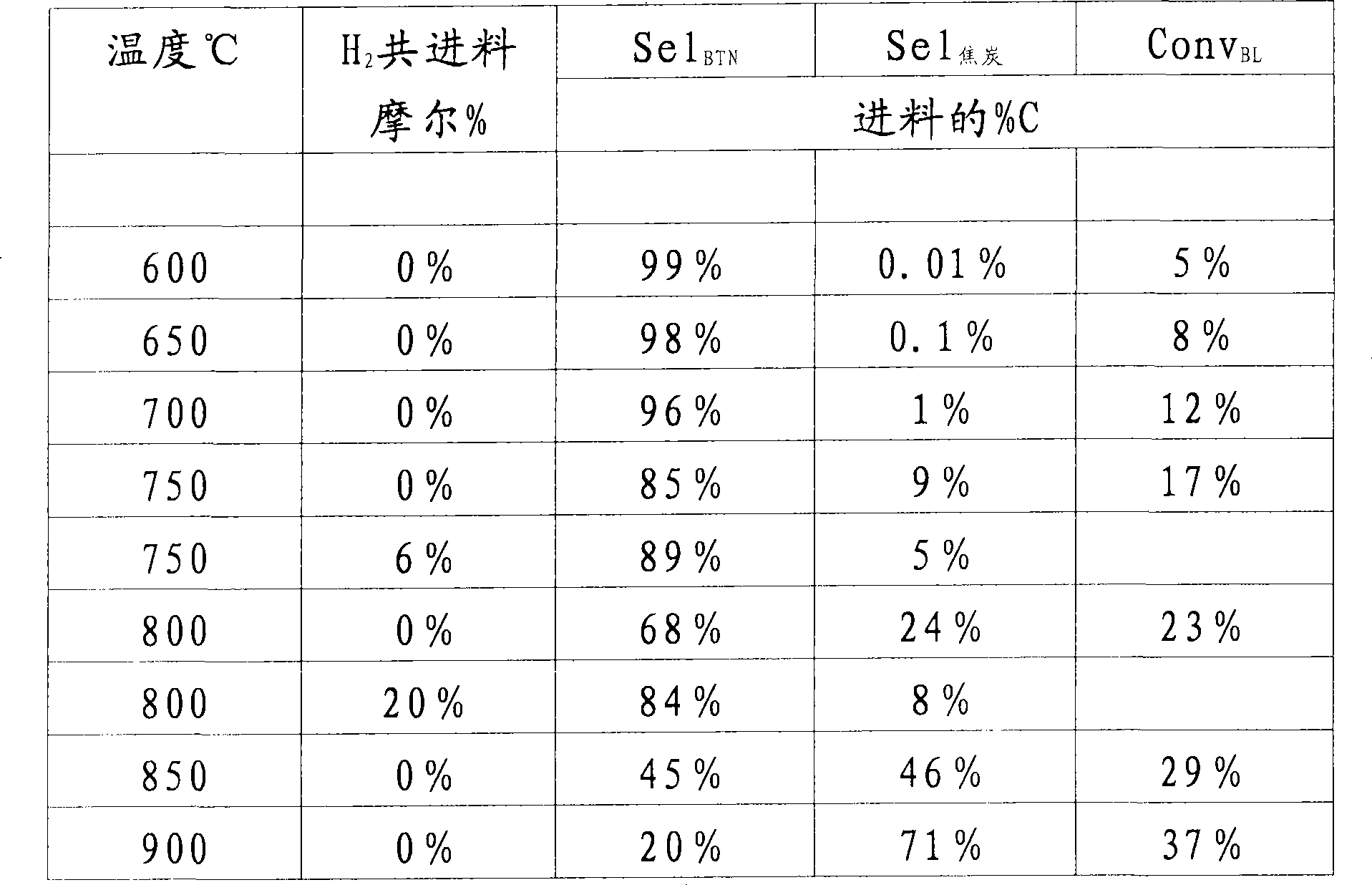
[0225] Catalytic implementation of methane dehydrocyclization to benzene using 95 wt.% CH 4 - 5 wt.% argon feed (argon used as internal standard) at different temperatures with a weight hourly space velocity (based on me...
Embodiment 2
[0267] Based on the model expected to favor the inverse temperature distribution, a laboratory-scale unit was constructed to confirm the model results. While the model is oriented towards the operation of the reaction system as a settled bed, the laboratory reactor is a fixed bed of catalyst with an inverse temperature distribution, which is influenced by the use of external heaters. In all cases, the experimentally obtained conversions were lower than those predicted by the model. This result may be due to laboratory-scale experimental artifacts such as bed splitting and or / back mixing caused by the hydrodynamic conditions in the laboratory-scale reactor operation.
[0268] Molybdenum / ZSM-5 catalyst via MoO with 7.5 wt% molybdenum (metal wt% based on the total weight of the catalyst) 3 with NH 4 ZSM-5 carrier (Si / Al 2 ratio of 25) ball milling for 2 hrs, followed by calcination in air at 500°C for 5 hrs. The catalyst was granulated, crushed and then sieved to a 20-40 mesh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Thermal diffusivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com