Chloramphenicol ophthalmic preparation and method for preparing the same
An ophthalmic preparation and a technology for chloramphenicol, which are applied in the field of chloramphenicol ophthalmic preparations and their preparation, can solve the problems of complex packaging, high glycol content, poor solubility of chloramphenicol, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
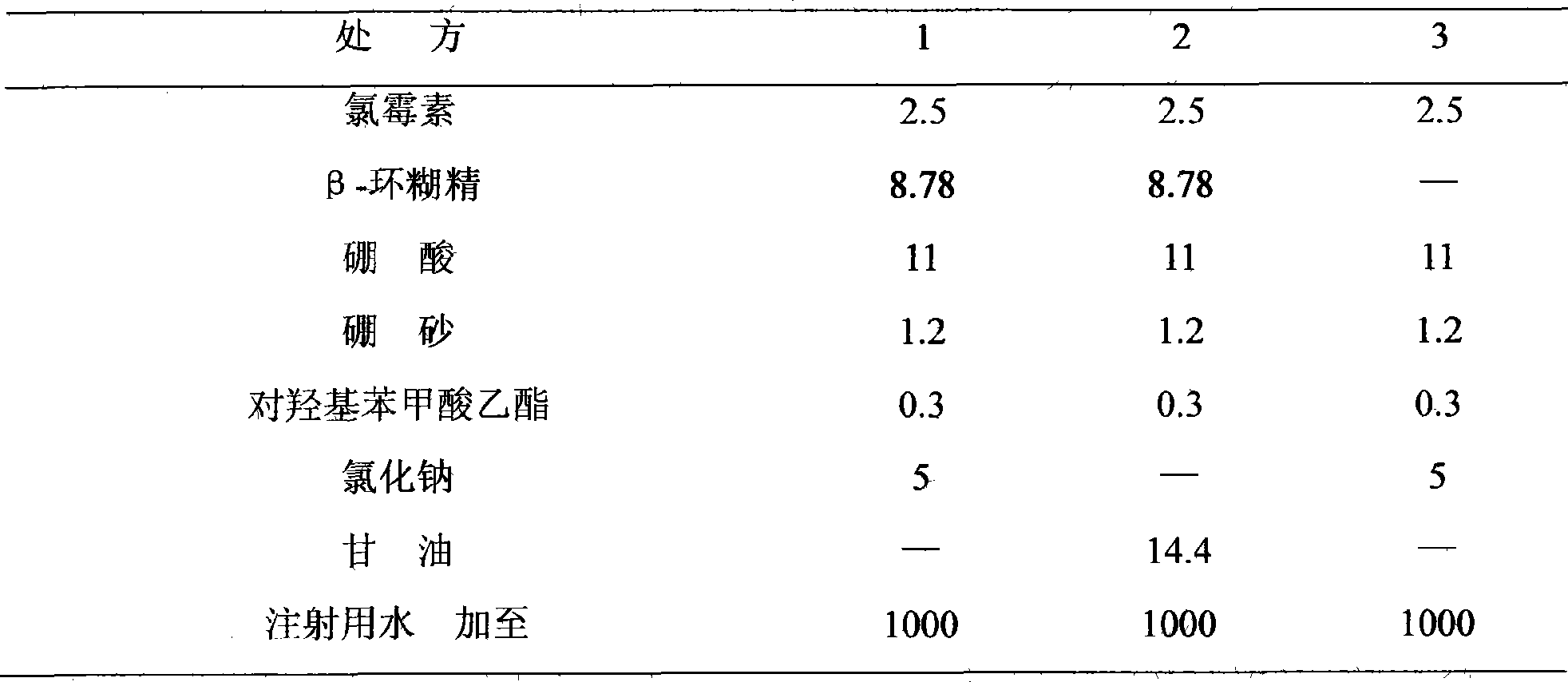
[0011] prescription:
[0012] β-cyclodextrin 8.78g
[0013] Chloramphenicol 2.5g
[0014] Boric acid 11g
[0015] Borax 1.2g
[0016] Ethyl p-hydroxybenzoate 0.3g
[0018] Add water for injection to 1000ml
[0019] Preparation:
[0020] 1. Take about 150ml of water for injection, put it in a 400ml beaker, heat it to 60°C, add β-cyclodextrin under stirring, after dissolving, add chloramphenicol under heat preservation and stirring, and continue heat preservation and stirring for 2 hours after dissolving to obtain β-cyclodextrin chloramphenicol mixed solution.
[0021] 2. Take 850ml of water for injection, add boric acid, borax, ethyl p-hydroxybenzoate and sodium chloride, heat and stir to dissolve, then add the above-mentioned β-cyclodextrin chloramphenicol mixed solution, heat and sterilize, cool, and set container, filter and fill.
Embodiment 2
[0023] prescription:
[0024] β-cyclodextrin 8.78g
[0025] Chloramphenicol 2.5g
[0026] Boric acid 11g
[0027] Borax 1.2g
[0028] Ethyl p-hydroxybenzoate 0.3g
[0030] Sodium Hyaluronate 1.0g
[0031] Add water for injection to 1000ml
[0032] Preparation:
[0033] 1. Take about 150ml of water for injection, put it in a 400ml beaker, heat it to 60°C, add β-cyclodextrin under stirring, after dissolving, add chloramphenicol under heat preservation and stirring, and continue heat preservation and stirring for 2 hours after dissolving to obtain β-cyclodextrin chloramphenicol mixed solution.
[0034] 2. Take 400ml of water for injection, add sodium hyaluronate to soak to dissolve it, take another 450ml of water for injection, add boric acid, borax, ethyl p-hydroxybenzoate and sodium chloride, heat and stir to dissolve, heat to sterilize, and cool to about 60°C, then add the above-mentioned β-cyclodextrin chloramphenicol mixed solution and so...
Embodiment 3
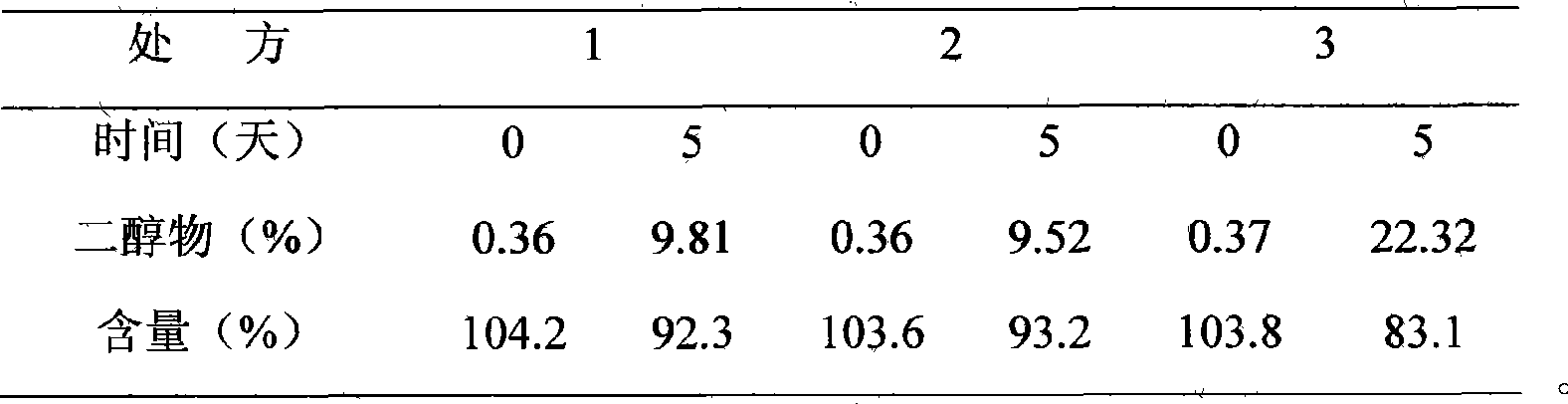
[0036] Take 1000ml of water for injection, put it in a 2000ml beaker, and heat it to 60°C; weigh 100g of β-cyclodextrin, and add it under stirring; then weigh 28.4g of chloramphenicol, add it into the above solution under stirring, and continue stirring , make it completely dissolved, continue to stir for 2 hours under heat preservation, let it cool, place it at 0-5°C for about 10 hours, filter to obtain a mixture of β-cyclodextrin and chloramphenicol, dry it in vacuum at low temperature, and use high-efficiency liquid phase The chloramphenicol content was determined to be 20.66%. Then prepare chloramphenicol eye drops according to the following prescription and preparation method:
[0037] prescription:
[0038] β-cyclodextrin chloramphenicol mixture 12.1g (containing chloramphenicol 2.5g)
[0039] Boric acid 11g
[0040] Borax 1.2g
[0041] Ethyl p-hydroxybenzoate 0.3g
[0042] Sodium chloride 5g
[0043] Add water for injection to 1000ml
[0044] Preparation:
[004...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com