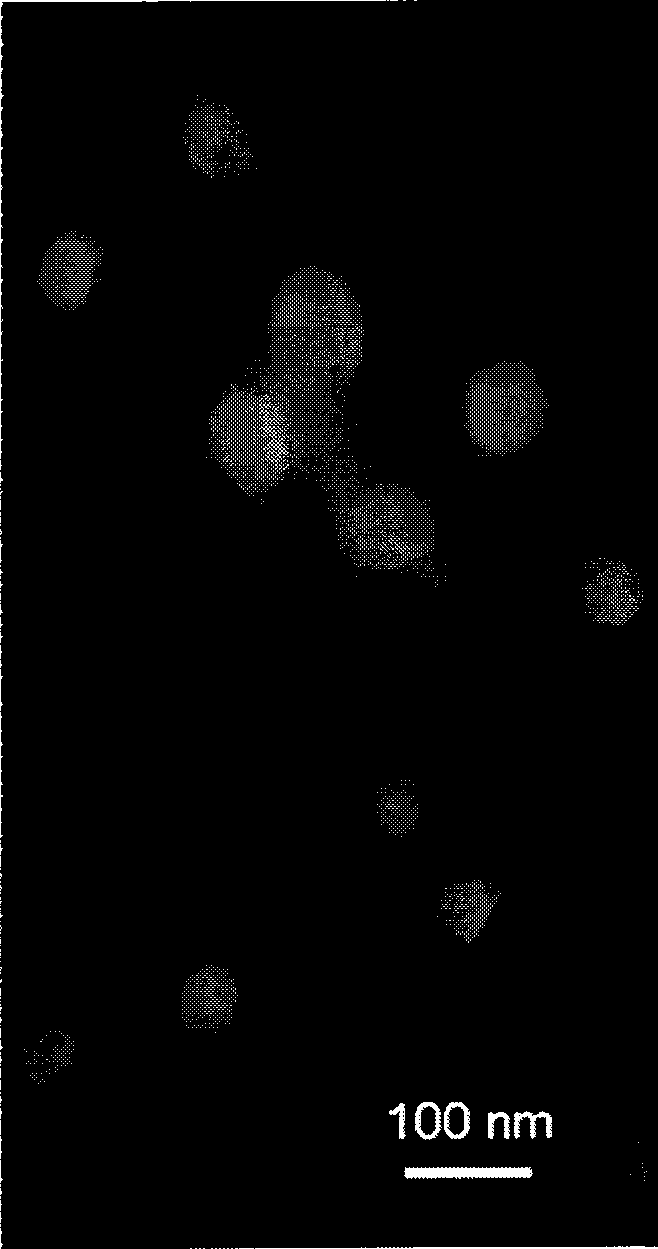Novel cation lipoid, preparation and use thereof
A cationic lipid and solid lipid technology, applied in the chemical field, can solve the problems of high toxicity, affecting cell resistance to gene transfection, etc., and achieve the effects of small particle size, improved gene therapy effect, and strong binding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
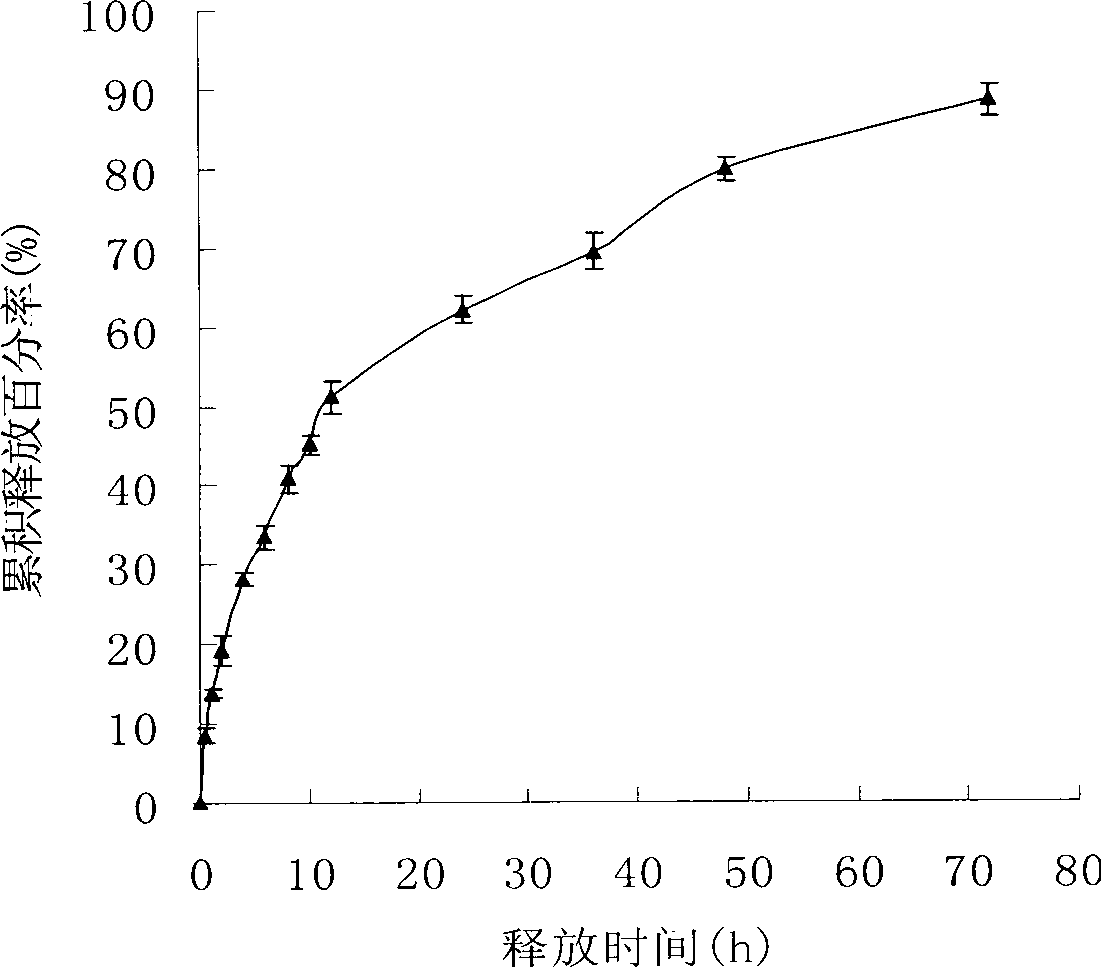
Image
Examples
Embodiment 1
[0052] Embodiment 1. the synthesis of 6-(2,6-diamino-hexanoyloxy)hexyl dodecanoate (LHLN)
[0053] Weigh 15 g (about 0.1 mol) of lysine (Lys), add 200 mL (0.2 mol) of 1 mol / L sodium hydroxide to dissolve, add dropwise the amino protecting agent di-tert-butyl dicarbonate (Boc) dissolved in tetrahydrofuran under ice bath , control the pH value to 8-9, and stir the reaction at room temperature for 24 hours. After extraction, the water was evaporated to dryness under reduced pressure to obtain lysine (Boc-Lys) protected by di-tert-butyl dicarbonate. Weigh 35 g (about 0.1 mol) of lysine (Boc-Lys) protected by di-tert-butyl dicarbonate and mix with 11.8 g (0.1 mol) of hexanediol, dissolve in anhydrous dichloromethane, add catalyst 4-dichloromethane under ice bath 2.44 g (0.02 mol) of methylaminopyridine (DMAP) and 22.66 g (0.11 mol) of dehydrating agent N, N'-dicyclohexylcarbodiimide (DCC), were stirred and reacted at room temperature for 24 hours. After extraction, the water was ...
Embodiment 2
[0056] Embodiment 2. Synthesis of 6-(2,6-diamino-hexanoyloxy)hexyl myristate
[0057] Weigh 15 g (about 0.1 mol) of lysine (Lys), add 200 mL (0.2 mol) of 1 mol / L sodium hydroxide to dissolve, add dropwise the amino protecting agent di-tert-butyl dicarbonate (Boc) dissolved in tetrahydrofuran under ice bath , control the pH value to 8-9, and stir the reaction at room temperature for 24 hours. After extraction, the water was evaporated to dryness under reduced pressure to obtain lysine (Boc-Lys) protected by di-tert-butyl dicarbonate. Weigh 35 g (about 0.1 mol) of lysine (Boc-Lys) protected by di-tert-butyl dicarbonate and mix with 11.8 g (0.1 mol) of hexanediol, dissolve in anhydrous dichloromethane, add catalyst 4-dichloromethane under ice bath 2.44 g (0.02 mol) of methylaminopyridine (DMAP) and 22.66 g (0.11 mol) of dehydrating agent N, N'-dicyclohexylcarbodiimide (DCC), were stirred and reacted at room temperature for 24 hours. After extraction, the water was evaporated to...
Embodiment 3
[0060] Embodiment 3. Synthesis of hexadecanoic acid 8-(2,6-diamino-hexanoyloxy) octyl ester
[0061] Weigh 15 g (about 0.1 mol) of lysine (Lys), add 200 mL (0.2 mol) of 1 mol / L sodium hydroxide to dissolve, add dropwise the amino-protecting agent di-tert-butyl dicarbonate dissolved in tetrahydrofuran under ice bath (Boc), control pH value 8-9, stir reaction at room temperature for 24 hours. After extraction, the water was evaporated under reduced pressure to obtain lysine (Boc-Lys) protected by di-tert-butyl dicarbonate (Boc). Weigh 35g (Boc-Lys) of lysine (Boc-Lys) protected by di-tert-butyl dicarbonate and mix with 14.6g (0.1mol) of octanediol, dissolve in anhydrous dichloromethane, add catalyst 4- Dimethylaminopyridine (DMAP) 2.44g (0.02mol) and dehydrating agent N,N'-dicyclohexylcarbodiimide (DCC) 22.66g (0.11mol) were stirred and reacted at room temperature for 24 hours. After extraction, the water was evaporated to dryness under reduced pressure. Then add 25.6 g (0.1 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com