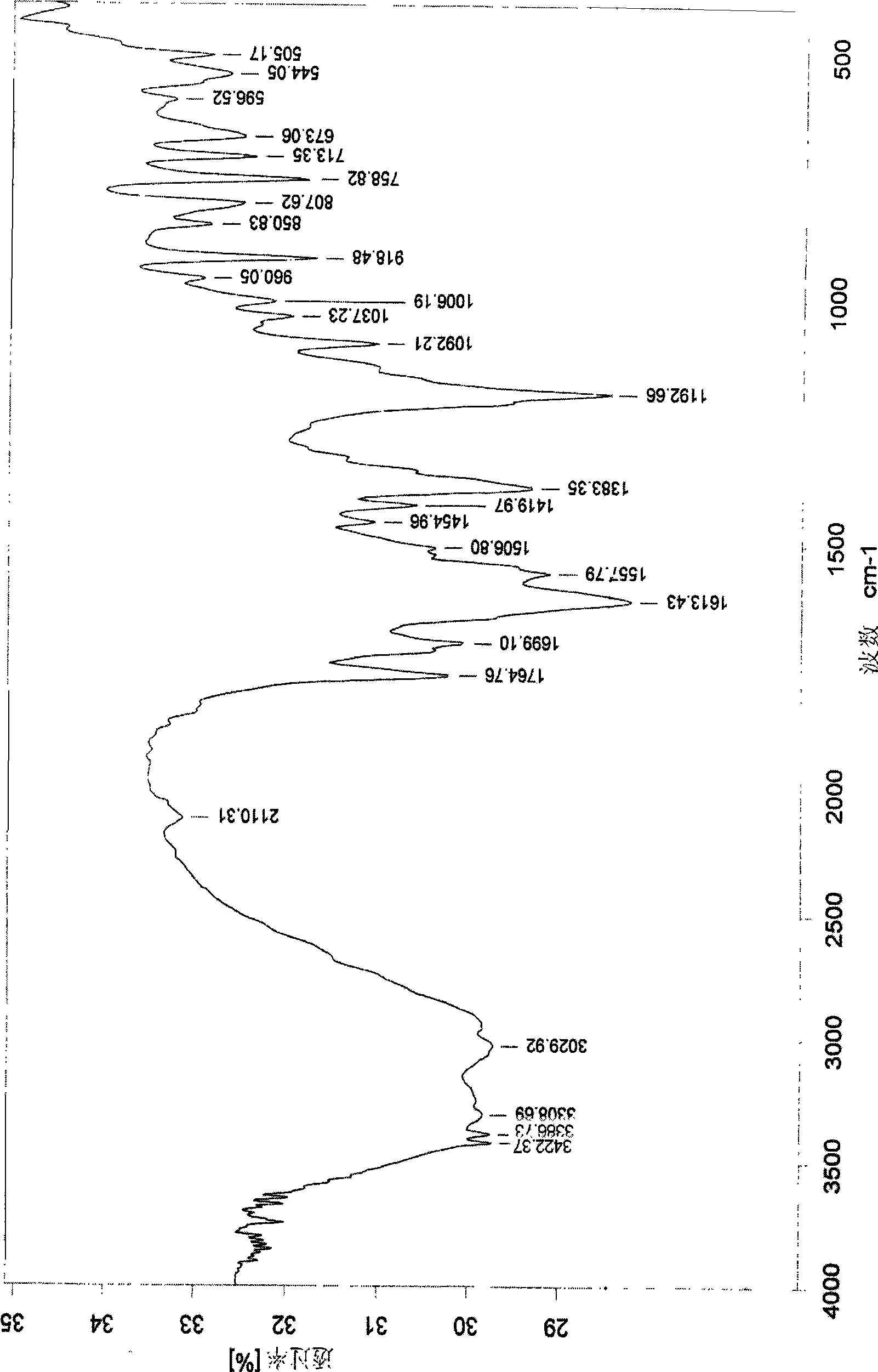
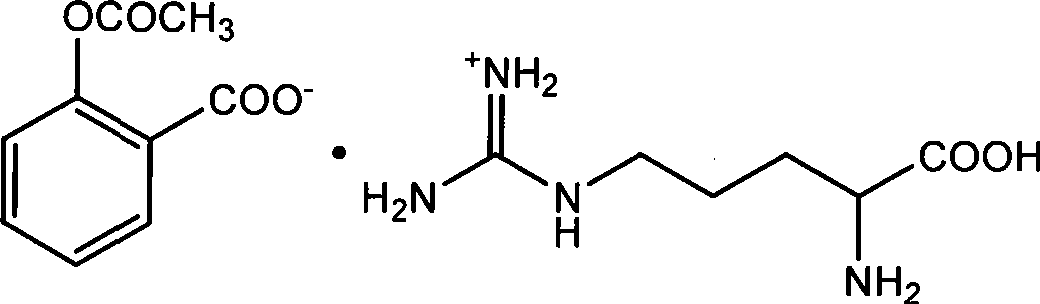
Preparation of arginine acetylsalicylate
An aspirin arginine salt and aspirin technology are applied in the field of preparation of aspirin arginine salt, can solve the problems of unfavorable industrialized production, complicated operation process and high equipment requirements, achieve good water solubility, simple reaction conditions and wide application range Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
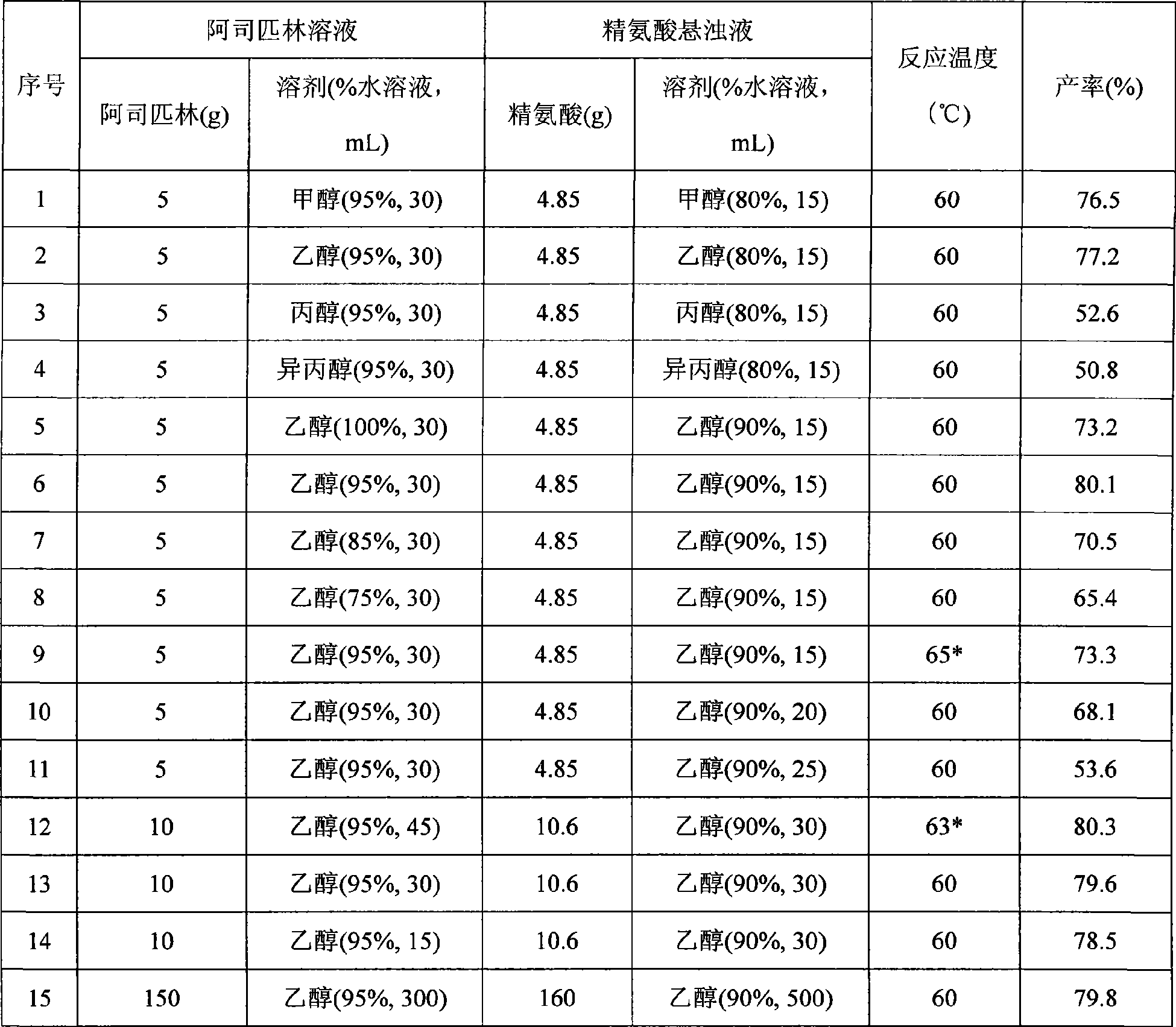
[0033] Dissolve 5 grams of aspirin in 30ml of 95% ethanol, suspend 4.85 grams of arginine in 15ml of 80% ethanol, add dropwise at a rate of 10mL / min, and add the aspirin solution dropwise to the essence at 60°C. In the amino acid suspension, after the dropwise addition, the arginine solid disappears, the reaction solution is clear, and cooled under stirring. After 10 minutes, the aspirin arginine salt precipitates, and the stirring is continued until about 30°C to stop. Place in the refrigerator overnight, filter with suction, collect the product, wash with 95% ethanol three times, each time the amount of 95% ethanol is 20 mL, and dry at 40°C. 7.6 g of product were obtained. Product yield calculated as aspirin: 77.2%, percent content: aspirin 53.58%, arginine 46.36%.
Embodiment 2
[0035] Dissolve 10 grams of aspirin in 45 ml of 95% ethanol, suspend 10.6 grams of arginine in 30 ml of 90% ethanol, add dropwise at a rate of 10 ml / min, add the aspirin solution dropwise to arginine at 63°C In the acid suspension, after the dropwise addition, the arginine solid disappears, the reaction solution is clear, and cooled under stirring. When the temperature drops to 42°C, aspirin arginine salt precipitates, continue to stir until about 30°C and stop. Place in a refrigerator at 0°C overnight, filter with suction, collect the product, wash with 95% ethanol three times, each 95% ethanol dosage is 30 mL, and dry at 40°C. 15.8 g of product were obtained. Product yield calculated as aspirin: 80.3%, percent content: aspirin 53.67%, arginine 46.13%.
[0036] Table 1 List of aspirin arginine salts prepared under different conditions
[0037]
[0038] * It is the product of free salicylic acid exceeding the standard
[0039] Table 1 is the aspirin arginine salt prepar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com