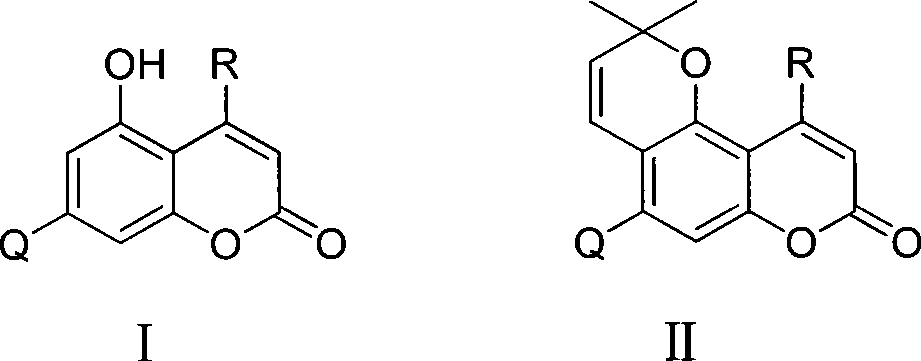
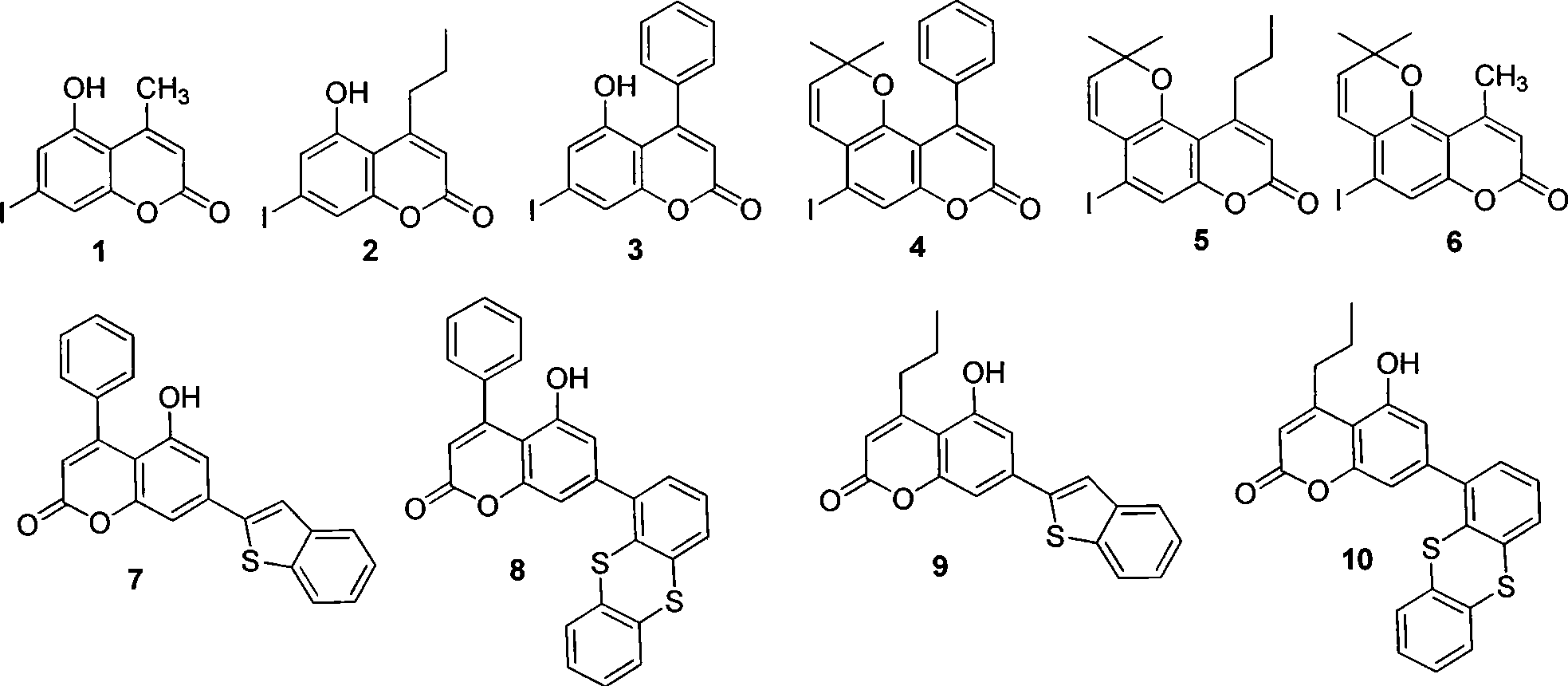
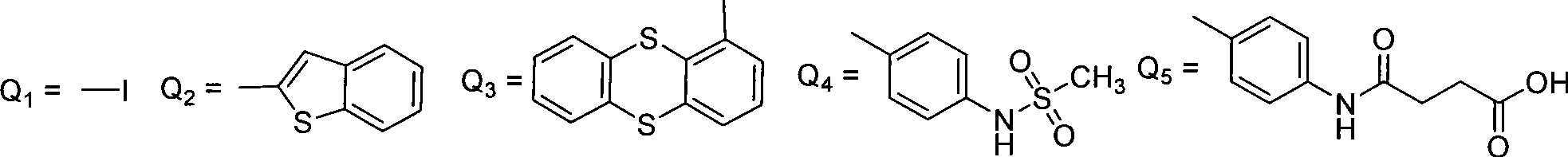5-hydroxy coumarin and pyranoid type coumarin compounds, synthesizing method and use
A technology of hydroxycoumarins and coumarins, which is applied in the directions of active ingredients of heterocyclic compounds, medical preparations containing active ingredients, and organic chemistry, can solve problems such as liver poisoning, achieve high yield, mature method, The effect of high therapeutic factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of Compound 1
[0030] Add 0.52 g of 5-iodo-1,3-benzenediol, 0.28 g of ethyl acetoacetate, silicon sulfonic acid (SO 2 -OSO 3 H) 0.9 g (equivalent to 2.3 mmol H + ), and added 10 ml of dichloromethane, and refluxed for 6 hours. After the reaction was complete, dissolve with hot ethanol and filter to remove silicon sulfonic acid, recrystallize from ethanol to obtain white crystal compound 1, yield 94.1%, melting point: 262 ℃ of decomposition, mass spectrum (APCI-MS): 302.6 (calculated value: 302.07 ).
Embodiment 2
[0031] Example 2 Preparation of Compound 2
[0032] Add 1.18 grams of 5-iodo-1,3-benzenediol, 0.79 grams of ethyl butyryl acetate, silicon sulfonic acid (SO 2 -OSO 3 H) 1.9 g (equivalent to 4.8 mmol H + ), and added 10 ml of dichloromethane, and refluxed for 6 hours. After the reaction is complete, dissolve with hot ethanol and filter to remove silicon sulfonic acid, recrystallize from ethanol to obtain white crystal compound 2, yield: 90.2%, melting point: 232-233 ° C, mass spectrum (APCI-MS): 330.7 (calculated value: 330.7).
Embodiment 3
[0033] Example 3 Preparation of Compound 3
[0034] Add 1.18 grams of 5-iodo-1,3-benzenediol, 0.96 grams of ethyl benzoylacetate, silicon sulfonic acid (SO 2 -OSO 3 H) 1.9 g (equivalent to 4.8 mmol H + ), and added 10 ml of dichloromethane, and refluxed for 8 hours. After the reaction is complete, dissolve with hot ethanol and filter to remove silicon sulfonic acid, recrystallize from ethanol to obtain light yellow crystal compound 3, yield: 89.0%, melting point: 240-241 ° C, mass spectrum (ESI-MS): 362.8 [M-H] + (calculated value: 364.13).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com